Vetter Development Services Rankweil is poised for expansion
Vetter’s Newest Clinical Manufacturing Site Successfully Completed its First Customer Fills
With the realization of the first successful customer fills, Vetter, a leading global Contract Development and Manufacturing Organization (CDMO) have reached another milestone at its new clinical manufacturing facility in Rankweil, Austria.
Further batches are already planned, and more customer projects are in the business pipeline. In addition, the site is being expanded to optimize processes and increase production capabilities.
 © Vetter Pharma International GmbH: Area for material preparation at Vetter's Rankweil site.
© Vetter Pharma International GmbH: Area for material preparation at Vetter's Rankweil site.
Within one year of its purchase, the site was adapted and integrated to meet Vetter’s high-quality standards. In December of 2021, successful completion of cGMP inspection by the responsible national regulatory authority, the Austrian Agency for Health and Food Safety (AGES) was achieved thus allowing the facility to support clinical development projects of global pharmaceutical and biotech companies.
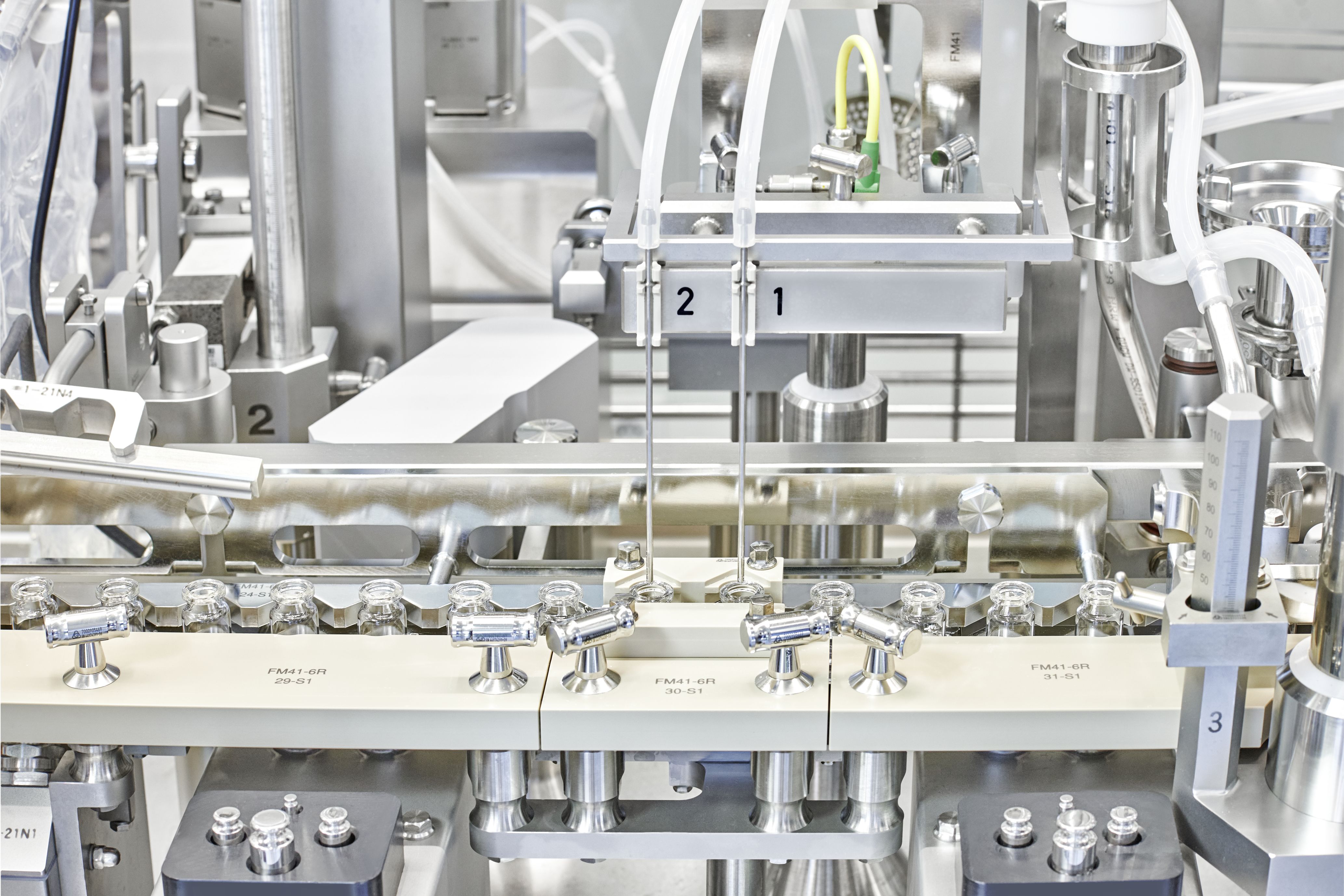 © Vetter Pharma International GmbH: Vial filling station at Vetter's Rankweil site
© Vetter Pharma International GmbH: Vial filling station at Vetter's Rankweil site
“We have been highly focused on achieving the strategic integration of the site,” said Dr. Martin Schwab, Site Head Austria. “With hard work and focus, we have managed to get the site fully operational quickly and are already planning for expansion and further development."
In order to offer customers additional production capacities, a second autoclave and another washing machine for material preparation will be implemented in the next few months, as well as a modification of the current lyophilizer to become consistent with its Ravensburg facilities, allowing for an optimized aligning of systems and processes. This will support for instance the transition from clinical to commercial production within Vetter.
Andrea Wesp, Vice President of New Business Development added, "Our intent is to support global customer projects as best as we can by offering a flexible and holistic service portfolio for clinical manufacturing. The positive feedback we have received from customers so far who have already visited our Rankweil site is not only proof that we are meeting their needs but that we have achieved an important step in our business strategy."
About Vetter
Headquartered in Ravensburg, Germany, Vetter is a family-owned, global leading contract development and manufacturing organization (CDMO) with production facilities in Germany, Austria, and the United States. Currently employing more than 5,700 individuals worldwide, the company has long-term experience in supporting biotechnology and pharmaceutical customers both large and small.
Vetter services range from early-stage development support including clinical manufacturing to commercial supply and numerous packaging solutions for vials, syringes, and cartridges. As a leading solution provider, Vetter appreciates its responsibility to support the needs of its customers by developing devices that contribute to increased patient safety, convenience, and enhanced compliance. Great importance is also given to social responsibility including environmental protection and sustainability.
