When coronavirus vaccines available for use?
 |
| Illustrative photo. |
| - Time to get a COVID-19 vaccine to market is likely to be at least 18 months. - Potential treatments require testing and funding. - International organizations are helping to fund research. |
COVID-19 is new and scientists understand little about how it behaves and spreads. The cost of creating a vaccine to protect people against the new coronavirus will run into billions of dollars and could take many months. Here are some of the reasons why.
Vaccines are complex to make
Technology is enabling new methods of exploring vaccine candidates for trial, but there are already a few tried and tested ways to make them.
In all of them, scientists try to stimulate the body’s immune system to combat invasive pathogens. That’s commonly done by creating something so similar to the pathogen that the body begins to create antibodies to fight off the real thing.
The most common way of doing this is to make what’s called attenuated vaccines – those that are made of weaker strains of the actual pathogen. Reared on animal cells outside of human bodies (some flu vaccines are cultured on chicken eggs), they are then extracted and injected in a single tiny dose.
Vaccines for measles and tuberculosis are created in this way.
Coronavirus china virus health healthcare who world health organization disease deaths pandemic epidemic worries concerns Health virus contagious contagion viruses diseases disease lab laboratory doctor health dr nurse medical medicine drugs vaccines vaccinations inoculations technology testing test medicinal biotechnology biotech biology chemistry physics microscope research influenza flu cold common cold bug risk symptomes respiratory china iran italy europe asia america south america north washing hands wash hands coughs sneezes spread spreading precaution precautions health warning covid 19 cov SARS 2019ncov wuhan sarscow wuhanpneumonia pneumonia outbreak patients unhealthy fatality mortality elderly old elder age serious death deathly deadly
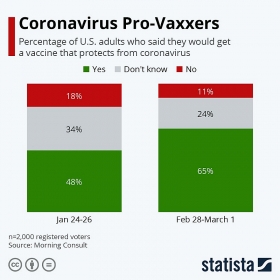
The number of US adults who would be vaccinated against COVID-19 grew between February and March.
Inactivated vaccines, on the other hand, are derived from identifying the active proteins in a virus that enables them to invade human cells. That’s done by taking dead samples of the pathogen and studying their genetic make-up so that scientists can replicate them en masse. When injected into a human, the body gets to work again constructing the necessary antibodies.
Often such vaccines require multiple doses over time, including those to protect against such diseases as rabies and polio.
Then there are nucleotide-based vaccines, which seek to replicate the genetic material – DNA and RNA – of a virus. This method stimulates the body’s own creation of this viral material in order for it to produce the necessary antibodies.
Most of the vaccines we rely on today took between five and 15 years to perfect.
| |||
Vaccines need lots of tests
A DNA-based vaccine for Zika virus, which was declared a public health emergency by the World Health Organization in 2016, was ready for clinical trials seven months after it was designed, but that is unusual.
There are already at least 35 companies and academic institutions racing to make a COVID-19 vaccine, with at least four candidates in the animal-testing phase. And one will enter human trials soon. But that’s just one hurdle cleared.
As Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases in the US, said: “A vaccine that you make and start testing in a year is not a vaccine that’s deployable. [It will take] a year to a year and a half, no matter how fast you go.”
Vaccines must be rigorously tested to ensure they not only work but will not cause other dangerous side-effects.
The trial methodology consists of three phases:
1. Testing on a small number of healthy adults
2. Testing on a larger number of adults in an area where the disease has spread
3.Testing on thousands of people in an area where the disease has spread
Each of these steps can last between six and eight months, but even if the vaccine candidate gets that far – many are abandoned or fail before then – they must then be studied by regulators before approval is granted.
“Constricting the whole timeline of going from concept to a product that can be distributed into a year or two is really a herculean endeavour,” said Jon Andrus, an adjunct professor of global vaccinology and vaccine policy at the Milken Institute of Public Health at George Washington University.
The good news
Once a candidate vaccine passes through those hoops, the challenge is to produce it in the volume necessary to end a pandemic.
A number of organizations are helping to fund the process. Among them is Norway-based CEPI, the Coalition for Epidemic Preparedness Innovations.
It was first launched at the World Economic Forum’s Annual Meeting in Davos in 2017, with the intention of bringing together public, private, philanthropic and civil organizations to develop vaccines against epidemics.
Backed by the Bill & Melinda Gates Foundation, among other donors, in 2020 CEPI announced a new partnership to develop a vaccine for COVID-19.
It has issued an urgent call for $2 billion of new funding, to expand the number of vaccine candidates at the outset to increase the chances of success, and to fund the clinical trials.
“Our ambition is to have at least three vaccine candidates, which could be submitted to regulatory authorities for licensure for general use/use in outbreaks.”
| CEPI has already provided funding to several organizations and institutions working on vaccines, including: Inovio Pharmaceuticals: Its DNA-based vaccine has begun pre-clinical trials, meaning the company is ready to test its candidate on humans. GlaxoSmithKline: Developing a “molecular clamp” vaccine, which would contain the protein that enables COVID-19 to enter human cells. This method was worked up by the University of Queensland in Australia. Moderna: The US company has already begun testing an RNA-based treatment. |
It remains to be seen how long it will take until there is a workable vaccine against COVID-19. For the time being, the best way to ensure you reduce your risk of infection is to follow the World Health Organization’s advice on handwashing and social distancing.
 | Coronavirus Vaccine: Global Competition The United States, China and Europe are battling to be the first finder a cure, bringing a nationalist element to a worldwide crisis that could ... |
 | China claims that Coronavirus vaccine could be ready by April China has said that some vaccines for the novel coronavirus could be in clinical use next month as the number of global coronavirus cases soared past 100,000. Zheng Zhongwei, ... |
 | Volunteers offered £3,500 to be infected with coronavirus A new scientific study is asking members of the public to volunteer to be infected with a coronavirus in return for cash. |
Recommended
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World