AstraZeneca COVID-19 vaccine: 30 blood clots found in UK, 7 people died
"Out of the 30 reports up to and including 24 March, sadly 7 have died," the Medicines and Healthcare Products Regulatory Agency (MHRA) said in a statement sent to AFP.
The Medicines and Healthcare products Regulatory Agency (MHRA) says the risk of having this type of clot is "very small".
The UK cases were out of more than 18 million doses given as of 24 March.
 |
| Several European countries have paused their roll-out of the jab (Photo: Reuters) |
Fears over clots have led nations such as the Netherlands, Germany, France and Canada to restrict the jab's use, according to BBC.
The pharmaceutical company AstraZeneca has said international regulators had found the benefits of its jab outweighed risks significantly.
The MHRA, the European Medicines Agency (EMA) and the World Health Organization (WHO) have also said the benefits of taking the vaccine outweigh any risks, AFP reported.
The regulator said that investigations are underway to determine if there is a link or if the cases are a coincidence and reiterated that increased adverse reaction or ADR reports reflect the increase in vaccine deployment.
“The number and nature of suspected adverse reactions reported so far are not unusual in comparison to other types of routinely used vaccines. The overall safety experience with both vaccines is so far as expected from the clinical trials," it adds.
As an additional measure, the British Society for Haematology has issued new guidance for doctors, amid concerns cases of blood clotting could be linked to a condition known as thrombocytopenia, according to Livemint.
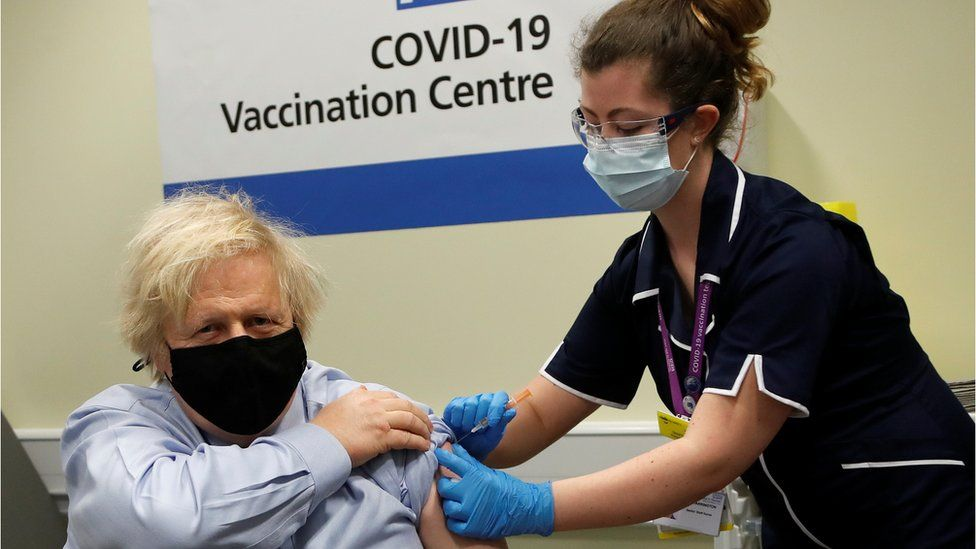 |
| Prime Minister Boris Johnson received the Oxford-AstraZeneca Covid vaccine last month (Photo: Reuters) |
“At the moment, any causal association with coronavirus vaccination has not been established. However, if you identify patients with this syndrome in proximity to coronavirus vaccination, it is very important that you complete the online Yellow Card – this will trigger a request from MHRA for further details," their guidance notes.
The Netherlands is the latest country to restrict the rollout of the Oxford-AstraZeneca jab, after saying on Friday that it was suspending its use for people under the age of 60, according to BBC.
The Dutch health ministry said the precautionary measure was taken after five reports of blood clots in combination with low platelet counts in women between the ages of 25 and 65.
All appointments for those under-60s scheduled to receive the jab have been cancelled with immediate effect. The suspension will remain in place until 7 April when the EMA is expected to issue new guidance.
About 400,000 Oxford-AstraZeneca injections have been given in the Netherlands, it added.
Earlier this week, Germany said it was suspending routine use of the jab for people below the age of 60 due to fears over a link with blood clots.
It has reported 31 CVSTs and nine deaths out of 2.7 million people vaccinated. Almost all the cases are reportedly in younger and middle-aged women.
The Netherlands and Germany were among several European countries which briefly suspended use of the jab last month pending an EMA review into the possible link to blood clots.
When the EMA declared the vaccine "safe and effective", they resumed its use while investigations continued.
France already limits use of AstraZeneca to those aged over 55 and on Monday, Canada recommended immediately suspending use of the jab in people aged below 55 following reports in Europe.
EMA backs AstraZeneca vaccine, but blood clot investigation ongoing
 |
| Photo: AFP |
There is "no evidence" to support restricting the use of the Oxford/AstraZeneca Covid-19 vaccine in any population, the head of the European Medicines Agency (EMA) has said.
The EMA said a causal link between unusual blood clots in people who have had the vaccine is "not proven, but is possible", adding that the benefits of the vaccine in preventing Covid-19 outweighed the risks of side effects.
It comes after it emerged Germany was suspending use of the Oxford/AstraZeneca vaccine for people aged under 60 due to fears of a link with rare blood clots, according to Telegraph UK.
Speaking at a press briefing, EMA executive director Emer Cooke said: "According to the current scientific knowledge, there is no evidence that would support restricting the use of this vaccine in any population."
Ms Cooke said 62 cases of cerebral venous sinus thrombosis (CVST) have been reviewed out of 9.2 million people in the European Economic Area (EEA).
The EMA said: "A causal link with the vaccine is not proven, but is possible and further analysis is continuing. "As communicated on March 18, EMA is of the view that the benefits of the AstraZeneca vaccine in preventing Covid-19, with its associated risk of hospitalisation and death, outweigh the risks of side effects."
Ms Cooke was asked if a link between the rare cases of blood clots and the vaccine is likely, and she said: "At the moment at this stage of our investigations the link is possible and we cannot say any more than that at this point."
 |
| Photo: CNN |
The EMA said vaccinated people should be aware of "the remote possibility of these very rare types of blood clots occurring", adding: "If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform healthcare professionals of their recent vaccination."
It comes as the German medicines regulator reported 31 cases of a type of rare brain blood clot among the nearly 2.7 million people who received the AstraZeneca jab in the country.
There have been moves in several German regions, including the capital Berlin, to stop using the vaccine in younger people.
Nine of the 31 people suffering clots have died, and all but two of the cases involved women who were aged 20 to 63, Germany's Paul Ehrlich Institute said. The two men were aged 36 and 57. Ms Cooke said the 62 figure she mentioned includes a "significant" number of the German cases but not all of them.
The concerns centre on CVST blood clots, which stop blood draining from the brain properly.
While a definitive link cannot be ruled out, senior regulators have said the benefits of having the vaccine far outweigh any potential risks and have declared it "safe and effective".
This view is echoed by the World Health Organisation, which has urged countries to continue using the jab. Covid itself can cause an increased risk of blood clots - a risk that is far higher than any posed by the vaccine.
 | Over 800 million Covax-sourced AstraZeneca vaccines arrive in Vietnam The first batch of Covid-19 vaccines sourced via the global Covax program, containing 811,200 AstraZeneca doses, has arrived at Noi Bai International Airport April 1 ... |
 | EU applied tough rules on vaccine exports, putting more pressures on AstraZeneca Strict rules on vaccine exports have been applied by The European Union, and also put more pressure on AstraZeneca to deliver more shots to the ... |
 | WHO confirms "no evidence of casuality caused by the vaccine" after examining AstraZeneca On Monday, The World Health Organization (WHO)'s advisory panel did a review on reports about AstraZeneca's COVID-19 vaccine that caused blood clots, and said there ... |
Recommended
 World
World
"Will continue offering our full support to Indian govt": US FBI Director after Pahalgam attack
 World
World
"Great Leader": JD Vance Lauds PM Modi During His India Visit
 World
World
Trump’s Tariff Pause: A Strategic Move from “The Art of the Deal”?
 World
World
"Indian Navy's participation in AIKEYME exercise matter of great happiness": Admiral Dinesh Kumar Tripathi
 World
World
ASEAN and US Tariff Dilemma: Hybrid Approach to Global Trade Tensions
 World
World
Vietnam Affirms Its Active and Responsible Role at UNESCO
 World
World
US Imposes 125% Tariff on China, Pauses Tariffs for 90 Days on Over 75 Countries
 World
World