China authorises emergency usage of select domestic Covid-19 vaccines
 |
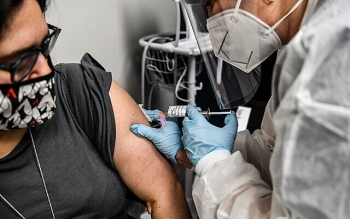
| An emergency use authorization of COVID-19 vaccine allows select unapproved vaccine candidates from domestic companies to be used among people who are at high risk of getting infected. (Photo: Today News) |
"We've drawn up a series of plan packages, including medical consent forms, side-effects monitoring plans, rescuing plans, compensation plans, to make sure that the emergency use is well regulated and monitored," Zheng Zhongwei, head of China's coronavirus vaccine development task force, told state-run CCTV on Saturday.
| According to China’s Law on Vaccine Management, when a very extreme public well being emergency happens, vaccines in medical trials can be utilized in a restricted scope to guard medical and epidemic prevention personnel, border officers and different individuals working in secure metropolis operations, Zheng mentioned. State-run Global Times has previously reported that employees of state-owned enterprises (SOEs) preparing to go abroad and frontline medics have been offered two choices of domestic inactivated vaccine candidates developed by Sinopharm for urgent use. On Thursday and Friday, Sinopharm signed cooperation agreements on phase III clinical trials of inactivated vaccines with Peru, Morocco and Argentina. Zheng noted that for the next step of preventing a possible outbreak this autumn and winter, vaccines' availability will be extended to people working in food markets, transport systems and services industries. The number of people being vaccinated on an urgent basis may reach hundreds of thousands across China, considering that personnel in wider sectors are being offered free injections, said Tao Lina, a Shanghai-based immunology expert, on Sunday. |
One month has handed since China formally launched the pressing use of COVID-19 vaccines on July 22, whereas the vaccines had been going by medical trials, Zheng mentioned.
Recipients who obtained their first dose since then revealed they’d few hostile reactions and none reported a fever, Today News Online said.
 |
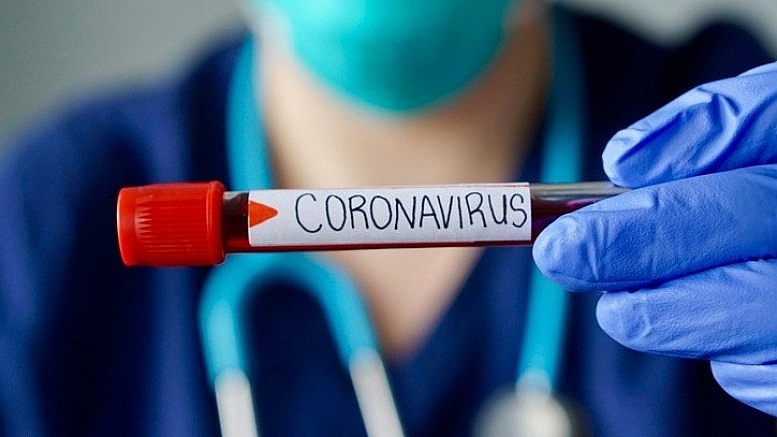
| Zheng Zhongwei, Head of China's Coronavirus vaccine Development Task force (Photo: CGTN) |
Wu, an employee of a state-owned company handling overseas construction projects along the Belt and Road Initiative (BRI) in Asian and African countries, told the Global Times on Sunday that all staff in her firm have been offered inactivated vaccine injections on a voluntary basis for free.
Wu, who took the vaccine on August 7 along with many of her colleagues, said she did not experience any adverse reactions, similar to everyone else in her group.
"My colleagues and I felt only a little dizzy on the afternoon of the vaccination, but we got over it pretty quickly. There was no local redness, swelling or pain, and we did not hear of anyone reporting a fever," said Wu, who will take her second dose on day 28 after the first shot”.
"People seem to be relaxed over the vaccination as most of us feel confident in domestically developed vaccines," she was quoted by Economic Times as saying.
One of Sinopharm's inactivated COVID-19 vaccines on August 13 was revealed to have had a low rate of adverse reactions for patients in phase I and II clinical trials, while also demonstrating immunogenicity results.
The inactivated vaccine might be efficient towards all detected strains of the virus not less than as of mid-July, with decrease probabilities and levels of hostile reactions than same-type vaccine candidates underneath analysis, Yang Xiaoming, head of Sinopharm, advised the Global Times in an earlier interview.
Yang mentioned on Saturday that greater than 20,000 individuals within the United Arab Emirates had taken inactivated COVID-19 vaccines developed by Sinopharm in section III medical trials, which have proven an excessive stage of security. The efficacy of the vaccine is underneath statement.
“The phase III trial in the UAE has had no reported cases of side effects so far,” Yang mentioned, including “volunteers joined faster than expected and the vaccine was well worth the wait”, as reported by Today News Online.
 | Sputnik V's third stage of trials can be called mass vaccination, said Russian developers The trials will be carried out in the Moscow Region and some 20,000 - 30,000 people will get the vaccine. |
 | Vaccine production: a billion-dollar industry According to estimates of research firm Alliance Bernstein (headquarter in the US), the global vaccine production industry is worth $ 52 billion per year. In ... |
 | Vietnam yet to purchase Russian Covid-19 vaccine, prepares to put indegious one on human trials The made-in-Vietnam vaccine has been confirmed to produce a good immune response against the novel coronavirus in animals and would be soon to put on . |
Recommended
 World
World
India reports 9 Pakistani Aircraft Destroyed In Operation Sindoor Strikes
 World
World
Thailand Positions Itself As a Global Wellness Destination
 World
World
Indonesia Accelerates Procedures to Join OECD
 World
World
South Korea elects Lee Jae-myung president
 World
World
22nd Shangri-La Dialogue: Japan, Philippines boost defence cooperation
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World