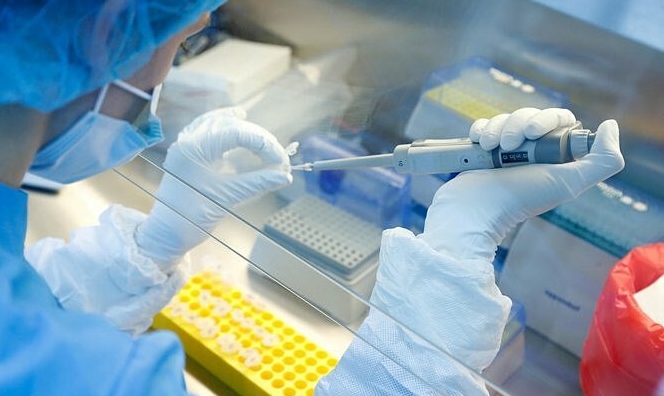
Sputnik V's third stage of trials can be called mass vaccination, said Russian developers
 |
| Sputnik V - COVID-19 vaccine production for civilian circulation to begin a month after the 3rd stage. Photo: Tass |
The third (post-registration) stage of research on the coronavirus vaccine Sputnik V, which is expected to be launched within 7-10 days, can be called mass vaccination because it will involve tens of thousands of people, Director of the N. F. Gamaleya Federal Research Center for Epidemiology and Microbiology of the Russian Health Ministry Alexander Gintsburg told TASS.
"According to the law, civilian circulation can begin now but the vaccine won’t be enough for both things [post-registration research and mass vaccination]. So, in the first place, the vaccine will enter post-registration research and this is also mass vaccination. It is called so because there is tougher control over those vaccinated. The civilian circulation [will start] when the demand for vaccine is met for post-registration [stage] - this is nearly 30,000 doses of drug, and then civilian circulation will begin immediately - with a delay of 3-4 weeks [since the start of the third stage]," the scientist said.
Gintsburg noted that the third stage could start within 7-10 days after the Health Ministry approves a certain protocol. The trials will be carried out in the Moscow Region and some 20,000 - 30,000 people will get the vaccine. "The post-registration research can take 4-5 months on average," he said.
On August 11, Russia registered the world’s first vaccine against the novel coronavirus. The vaccine, dubbed Sputnik V, was developed by the Gamaleya Research Institute of Epidemiology and Microbiology, and its clinical trials were successfully completed in June-July. It was created on a platform that had been used for the development of a number of other vaccines. On August 15, the Health Ministry announced that the production of the vaccine had been launched.
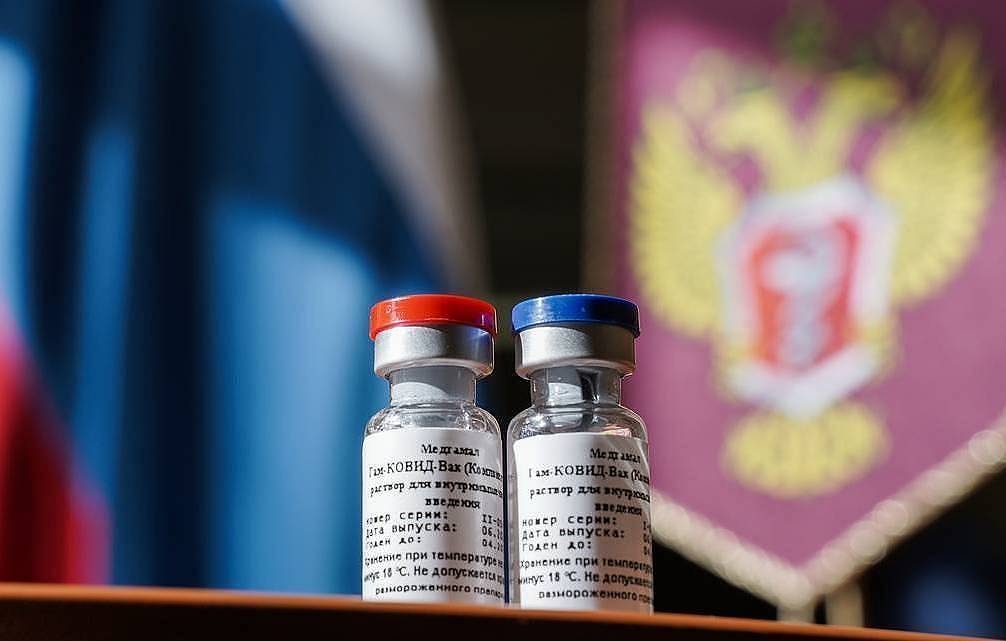
 |
| The coronavirus vaccine Sputnik V was launched on August 11. Photo: sputnikvaccine |
| The "Sputnik V" is a vaccine developed by the Gamaleya research institute in coordination with the Russian defence ministry. It is based on a proven vaccine against adenovirus - the common cold. The vaccine is expected to provide immunity from SARS-CoV-2, the virus that causes COVID-19, for up to two years, according to the Russian health ministry. But the results of the limited trials have yet to be made public. The vaccine is administered in two doses and consists of two serotypes of human adenovirus, each carrying an S-antigen of the new coronavirus, which enter human cells and produce an immune response. It is a so-called viral vector vaccine, meaning it employs another virus to carry the DNA encoding of the needed immune response into cells. Since phase 3 trials of this vaccine have not been completed yet, many scientists have expressed skepticism over its safety. The World Health Organization has said, cited by The Guardian that, all vaccine candidates should go through full stages of testing before being rolled out. Experts have said vaccines that are not properly tested can cause harm in many ways, from a negative impact on health to creating a false sense of security or undermining trust in vaccinations.
|
 | Vaccine production: a billion-dollar industry According to estimates of research firm Alliance Bernstein (headquarter in the US), the global vaccine production industry is worth $ 52 billion per year. In ... |
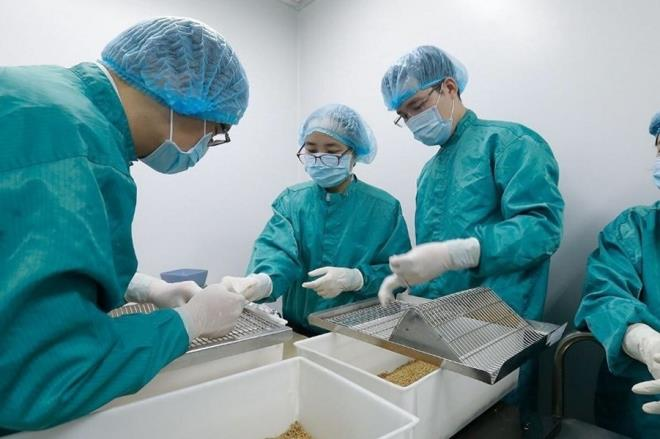 | Vietnam yet to purchase Russian Covid-19 vaccine, prepares to put indegious one on human trials The made-in-Vietnam vaccine has been confirmed to produce a good immune response against the novel coronavirus in animals and would be soon to put on ... |
 | Researchers determine materials making least effective masks for Covid-19 prevention Researchers at Duke University in the US launched an experiment to determine which types of masks are quite literally useless in shielding people from Covid-19 ... |
Recommended
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World
India strikes back at terrorists with Operation Sindoor
Popular article
 World
World
India sending Holy Relics of Lord Buddha to Vietnam a special gesture, has generated tremendous spiritual faith: Kiren Rijiju
 World
World
Why the India-US Sonobuoy Co-Production Agreement Matters
 World
World
Vietnam’s 50-year Reunification Celebration Garners Argentine Press’s Attention
 World
World