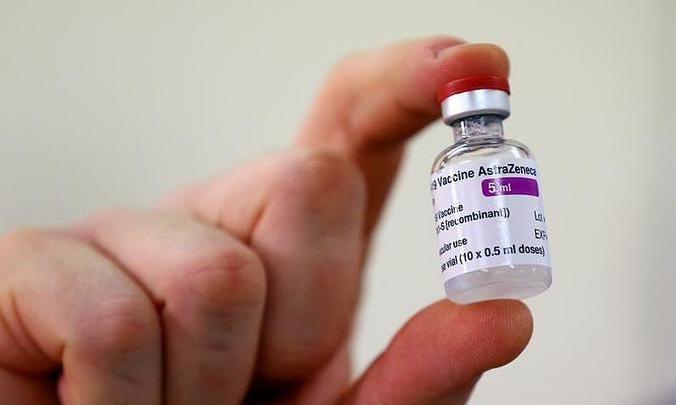
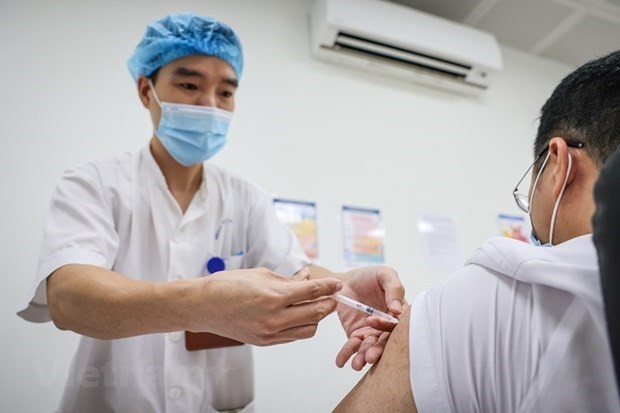
First batch of 204,000 Covid-19 vaccine doses to arrive in Vietnam on Feb 23
| Vietnam to receive 5 mln Covid-19 vaccine doses in February | |
| COVID-19 vaccinations in Japan to begin from Feb 17 | |
| 5 million COVID-19 vaccine doses set to be available in Vietnam late February |
This would be the first batch out of 30 million doses of vaccine that Vietnam had previously ordered to buy. The vaccine is developed by British-Swedish company AstraZeneca in collaboration with Oxford University, according to Vnexpress.
The Drug Administration of Vietnam under the Ministry of Health on Wednesday allowed AstraZeneca Vietnam Company Limited to import the doses under emergent circumstances to help fight against the Covid-19 pandemic which has turned more complicated in Vietnam.
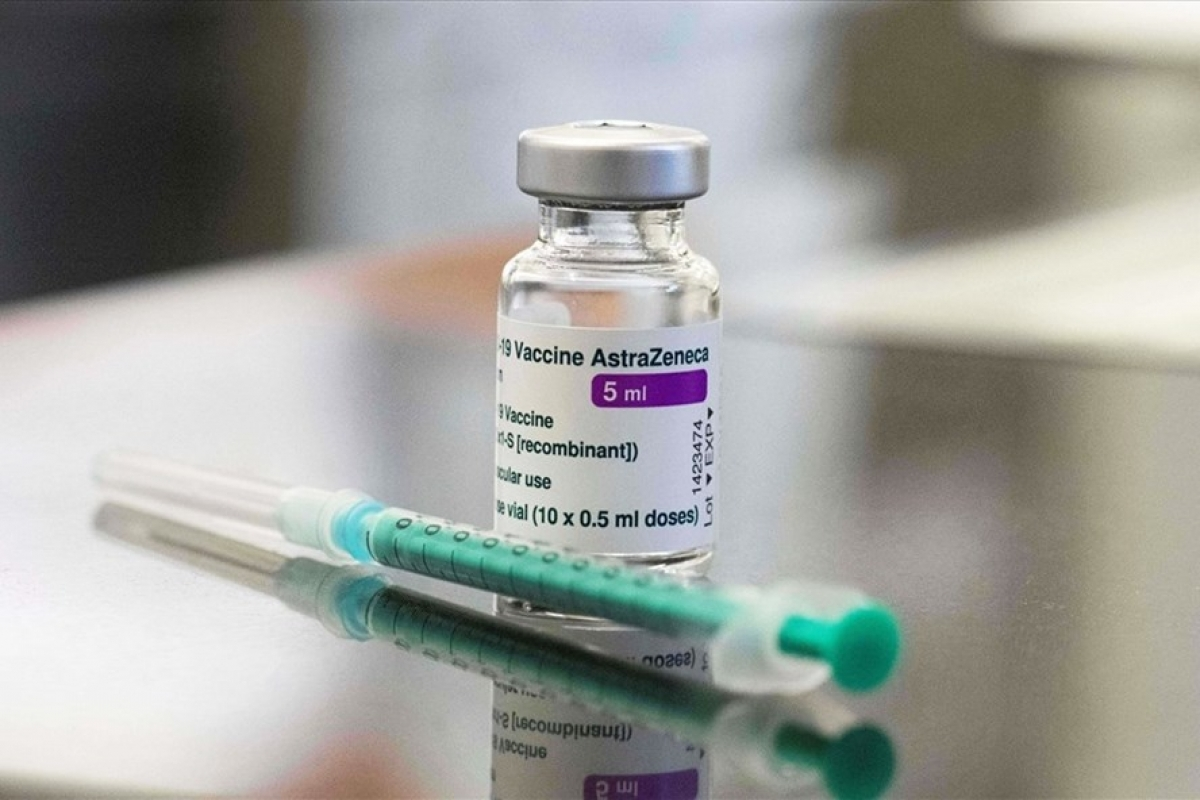 |
| 204,000 doses of the COVID-19 vaccine will be imported to battle a fresh wave of the coronavirus in Vietnam. Photo: AFP |
The health ministry on February 1 approved the AstraZeneca vaccine for domestic inoculation. Currently, this vaccine is licensed for one-year circulation or conditional import in the Philippines, Thailand, the U.K., Vietnam, and some other countries to help fight Covid-19.
The ministry earlier said doctors and other staff in frequent contact with Covid-19 patients or suspected infectees will be among the first to be vaccinated.
Other people on the priority list will be the elderly and those with chronic diseases that make them more vulnerable if they get infected, and officials in the diplomatic service.
Prime Minister Nguyen Xuan Phuc said importing Covid-19 vaccines should be a foremost priority of the government while calling for quick research progress on domestic Covid-19 vaccines as well.
Vietnam is also expected to receive 4.9 million vaccine doses by the end of this month, provided via Covax, a global mechanism for developing, manufacturing and procuring Covid-19 vaccine candidates and help member countries access vaccines as they become available.
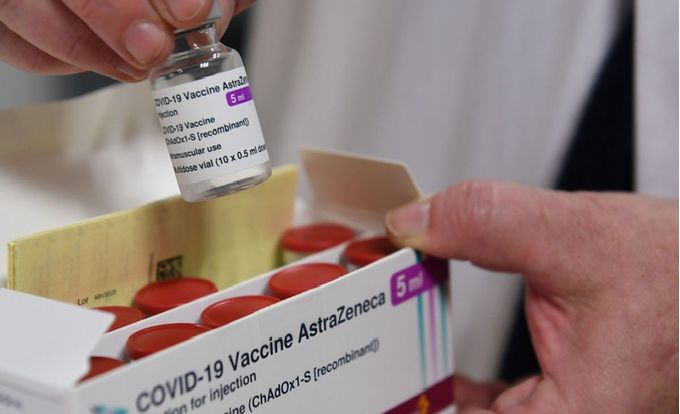 |
| A health worker opens a box containing the Oxford/AstraZeneca Covid-19 vaccine in France. Photo: AFP. |
Vietnam, the country’s been showered with praises over comprehensive and effective COVID-19 containment attainment is having four potential vaccines on hands. The vaccines are studied and produced by Nanogen, Vabiotech, Polyvac and the Institute of Vaccines and Medical Biologicals (IVMB), all have completed the laboratory production process.
While Vabiotech and Polyvac’s vaccines are still under evaluation on animals, IVMB’s candidate Covivac is scheduled to enter human trials this January after yielding safe and strong immunity response on animals.
As reported by VOV, IVAC will cooperate with the National Institute of Hygiene and Epidemiology and Hanoi Medical University to trial the vaccine on 125 volunteers aged 18-59. Those receiving IVAC’s trial jabs must be healthy, having no underlying disease and satisfying several other specific criteria.
In the meantime, Nanocogen’s Nonacovax vaccine finished its first phase of human trials. Nanogen Biopharmaceutical company is expected to end its Nanocovax vaccine’s human trials by February 2022.
The human clinical trials protocol, which includes three phrases, was approved by the Ethics Council of the Ministry of Health on December 9. Each phrase consists of two injections, 28 days apart. Nanocovax is priced at VND120,000 ($5.17) per dose. Along with injections, Vietnam’s COVID-19 Nanocovax vaccine will also be developed in the form of eye-drop and nasal spray for special subjects.
Vietnam has been struggling with a wave of Covid-19 outbreak since January 28 after 55 clean days in a row, with 737 community transmissions recorded in 13 localities, including major cities Hanoi and HCMC.
| On the global scale, there are currently 11 COVID-19 vaccine candidates under the third phase of human trials. Pfizer/BioNTech’s vaccine (the US) is the first vaccine to complete the trials with 95 percent effectiveness and granted emergency use authorization from the UK and Bahrain. Meanwhile, Moderna’s vaccine is in its final clinical trial phase, with an effective rate reaches 94.5 percent. Oxford/ AstraZeneca is 70-90 percent effective, depending on the injection dose. Russia’s Sputnik V (95 percent effective) is scheduled to begin mass vaccination next week. Moderna’s vaccine is priced at 37 USD per dose, meanwhile, Pfizer’s vaccine and Oxford’s vaccine are more reasonably priced at 19 USD and 3 UDD per dose, respectively. |
 | Prime Minister orders purchasing COVID-19 vaccine this month Prime Minister Nguyen Xuan Phuc has asked the health sector to conduct all necessary measures to make COVID-19 vaccines available in Vietnam this month either ... |
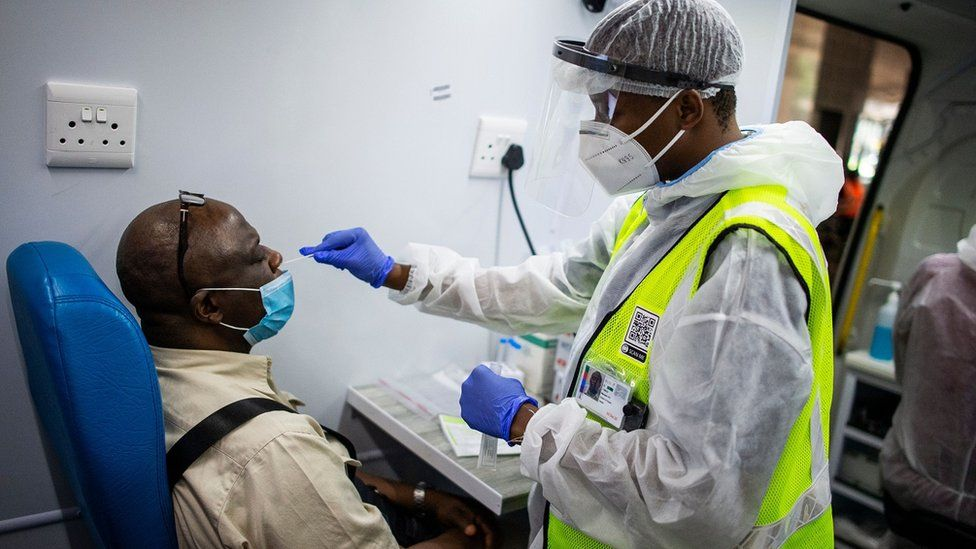 | South Africa's vaccine shots delayed and growing concerns of vaccine's effectiveness The country halted the vaccine shots after receiving the first batch of AstraZeneca vaccines, worrying about its effectivness with the new variant, and now struggling ... |
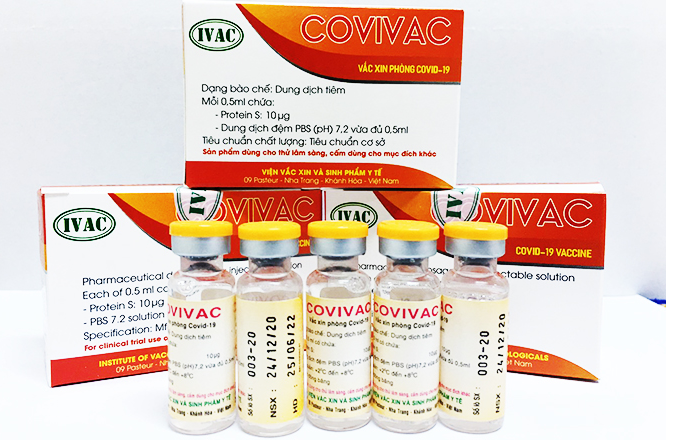 | Vietnam’s COVID-19 vaccine proves effective on new variants The locally-made Covivac vaccine is said to have triggered high immunity response against new Covid-19 variants emerging in the world, but further studies are still ... |
Recommended
 Viet's Home
Viet's Home
Vietnam Through Paintings of Overseas Children in Japan
 Viet's Home
Viet's Home
Uncle Ho through Documents and Artistic Creations
 Viet's Home
Viet's Home
Compassion House - John Donovan Project Supports 10 Great Solidarity Houses in Tien Giang
 Viet's Home
Viet's Home
Harnessing Emerging Technologies for Flood Risk Warning in Vietnam
Popular article
 Viet's Home
Viet's Home
Tzu Chi Grants Over VND 2 Billion to Support Disadvantaged Students in Hai Duong
 Viet's Home
Viet's Home
CARE Supports Hoa Binh Residents to Develop Sustainable Hemp Products
 Viet's Home
Viet's Home
"Ho Chi Minh Biography" in Greek Launched in Athens
 Viet's Home
Viet's Home