Vietnam’s Nanocovax COVID-19 vaccine generates high immunity response
 |
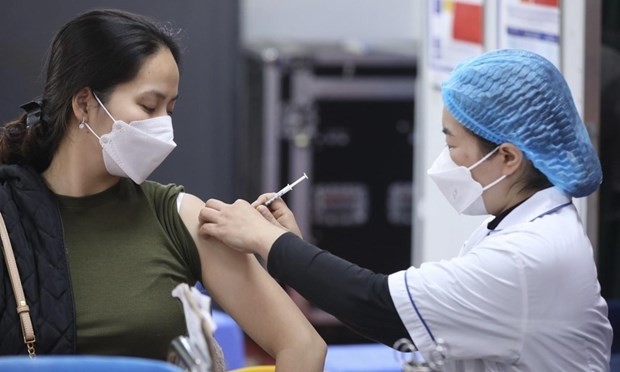
| One volunteer (in black shirt) getting the 25 mcg jab on January 14 (Photo: VNE) |
“This is the initial assessment from blood analysis of volunteers on day 7, 14, 21 after the first jab”, Associate Professor, Dr Ho Anh Son, Deputy Director of the Military Medical Research Institute, Military Medical Academy said. “To evaluate the durability and longevity of the antibody, the team will monitor the volunteers' health for another 6 months to a year”.
Vaccine research data is being processed to determine appropriate dose for the second phase. The data will also be reported to the Ministry of Health.
The second phase trial, with more extensive criteria, could start right after the Lunar New Year (mid-February) with two jabs. Over 560 volunteers are expected to be injected.
The Military Medical Academy on January 14 morning gave a second 25 mcg dose to three volunteers aged 25-42 who received the first dose last December 17.
Also on Thursday, another 10 volunteers were injected with the 75 mcg dose, one day earlier than expected. These volunteers are under further monitor at the Military Medical Academy, VNE reported.
 | |
|
Nanocovax, developed by Nanogen Biopharmar since May 2020, is the first locally-made vaccine candidate in Vietnam to enter human trials. To date, phase on of the trials has gone half the way with 60 volunteers got the injection.
The second phase is planned to start right after the first one and last for 6 months. 400-600 volunteers aged 12-75 will be recruited for the trial, which evaluates the immunogenicity and preventive potency of the vaccine.
The third phase (August 2021 - February 2022) is planned to take place in an epidemiological area in India, Indonesia, or Bangladesh to gather enough 1,500 – 3,000 volunteers aged 12-75.
Nanocovax is priced at VND120,000 ($5.17) per dose.
Along with injections, Vietnam’s COVID-19 Nanocovax vaccine will also be developed in the form of eye-drop and nasal spray for special subjects.
 | |
|
| In which: 25 mcg group: all volunteers received the first dose. On January 14, the first three people received a second dose. 50 mcg group: all volunteers received the first dose, waiting for the second dose. 75 mcg group: 13 people received the first dose and are monitoring after injection. The remaining 7 people, scheduled for injection on January 15. |
In Vietnam, the country’s been showered with praises over comprehensive and effective COVID-19 containment attainment is having four potential vaccines on hands. The vaccines are studied and produced by Nanogen, Vabiotech, Polyvac and the Institute of Vaccines and Medical Biologicals (IVMB), all have completed the laboratory production process.
While Vabiotech and Polyvac’s vaccines are still under evaluation on animals, IVMB’s candidate Covivac is scheduled to enter human trials this January after yielding safe and strong immunity response on animals.
As reported by VOV, IVAC will cooperate with the National Institute of Hygiene and Epidemiology and Hanoi Medical University to trial the vaccine on 125 volunteers aged 18-59. Those receiving IVAC’s trial jabs must be healthy, having no underlying disease and satisfying several other specific criteria.
According to Dr. Duong Huu Thai, Director of IVAC, the Institue has been studying Covivac since last May, aiming at successfully producing the vaccine and completing three human trials within 18 months. The first phase of the trails is scheduled to finish in April.
 | Vietnam's second COVID-19 vaccine set to enter human trials this month IVAC's Covivac COVID-19 vaccine will enter human trials in January after safety test assessments in animals, becoming the second vaccine in Vietnam to granted such ... |
 | Made-in-Vietnam COVID-19 vaccine promises a bright prospect Nanogen Biopharmaceutical company is expected to end its Nanocovax vaccine’s human trials by February 2022, opening hope that Vietnamese people will be resonably vacinated against ... |
 | Vietnam all geared up for COVID-19 vaccine trials The first 60 volunteers among the selected 200 will be injected with Nanocovax – Vietnam's first local-made COVID-19 vaccine approved for human trials, from Thursday. |
Recommended
 National
National
Shangri-La Dialogue 22: Vietnam Highlights Some Issues of Ensuring Stability in a Competitive World
 National
National
Vietnam News Today (Jun. 3): PM Pham Minh Chinh to Attend UN Ocean Conference, Visit Estonia, Sweden
 National
National
Vietnam News Today (Jun. 2): Vietnamese Trade Mission Sounds Out Business Opportunities in United States
 National
National
Vietnam News Today (Jun. 1): Vietnamese, Japanese Firms Foster Partnership
Popular article
 National
National
Vietnam News Today (May 31): Vietnam Strongly Supports Laos’s National Development
 National
National
Vietnam News Today (May 30): Vietnam, Venezuela Reinforce Ties Through People-to-people Diplomacy
 National
National
Vietnam News Today (May 29): Vietnam and Hungary to Expand Cooperation into New Areas
 National
National