Man who lost 7 relatives to COVID-19 becomes first American to trial Oxford vaccine
 |
| Man who lost 7 relatives to COVID-19 becomes the first American to trial Oxford vaccine (Photo: CBS News) |
Oxford University researchers have started the final round of testing for their COVID-19 vaccine in the U.S. — a significant leading step in the race to beat the coronavirus pandemic, CBS cited.
Oxford researchers, in partnership with AstraZeneca, started dosing the first volunteers in Florida on Friday, and 31 Americans received either the vaccine or placebo throughout the weekend, as part of the clinical trial.
During the third and final phase, the vaccine is tested for safety and how effective it is at reducing or blocking COVID-19 symptoms. The Food and Drug Administration would then consider approving it for public use.
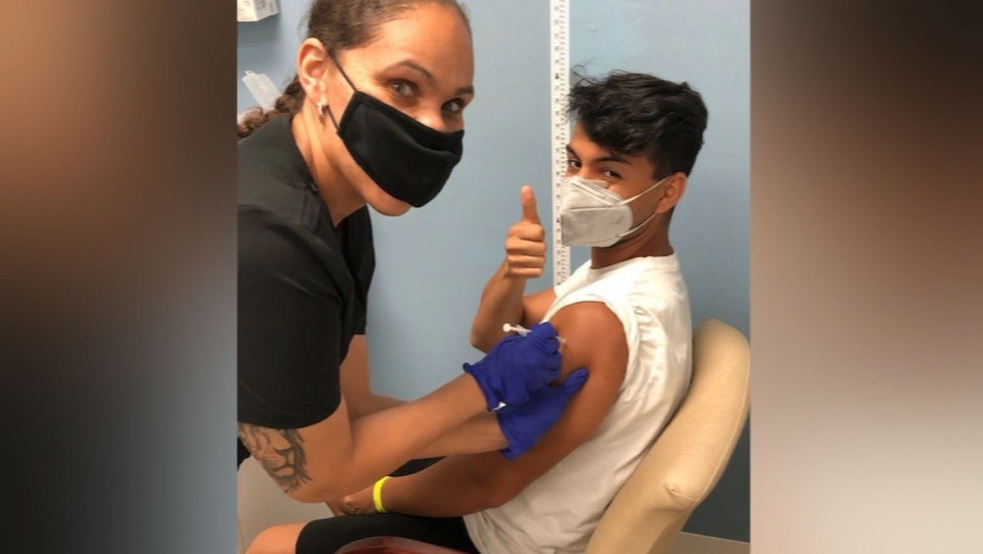
Jacob Serrano, 23 years old, is among 31 volunteers for a Florida trial of a potential vaccine developed by Oxford and AstraZeneca — and the first to try the drug when the final phase of US trials got underway on Friday, according to the New York Post.
Answering the CBS interview, Serrano said that the vaccine trial is a mission he wanted to fulfill after losing cousins, aunts, and uncles, a total of seven family members to the coronavirus.
“Each person that’s taken away from your life, it’s a big shift,” Serrano said.
 |
| The trial took place in Atlantis last Friday. (Photo: CBS News) |
The 23-year-old didn’t know what to expect when he arrived to do the study inside JEM Research Institute near JFK Medical Center Main Campus in Atlantis last Friday. He's confident he was injected with the Oxford University-AstraZeneca vaccine and not the placebo. He knew of the risks, but Serrano says it’s his way of being a part of the solution to save lives, no matter what it takes.
AstraZeneca researchers tested tens of thousands of volunteers overseas in places like the U.K. and South Africa, and are planning the same trials in Russia and Japan.
Dr. Larry Bush, the principal investigator to this Palm Beach County site, says he is hopeful.
“In phase one in two trials, the vaccine has been proven that not only do you get robust neutralizing antibodies to fight the coronavirus, you get a t-cell response, another arm of the immune system to fight off the cells that do become infected. That's crucial in treating infections,” Dr. Bush said.
The pharmaceutical company is expecting to have the trial results from this last stage later this year. AstraZeneca claims to have the capability of manufacturing two billion doses by the summer of 2021. The U.S. has reportedly already ordered 300 million doses.
| The Oxford team found that its vaccine provoked an immune response and didn’t produce any serious side effects. The vaccine prompted neutralising antibodies—the kind that defend cells against attack from virus—in at least nine out of ten of those who had a single dose of the vaccine, according to The wired. The immune response peaked 28 days after the vaccine, but it remained high until day 56, which was the final day covered by this scientific paper. The study is still ongoing. The results are from a study involving 1,077 healthy adults aged between 18 and 55. Half of the participants received the new Covid-19 vaccine, while the other half—the control group—received a vaccine against a bacterial infection. Although no serious side effects were reported, around 70 percent of participants developed either a fever or a headache, although this was lower in a subgroup of participants who took a paracetamol around the same time they had the vaccination. At this stage, the study can’t tell us whether people who have the vaccine are protected against contracting Covid-19, but it does tell us that the vaccine is safe to use and that it provokes an immune response. |
 | COVID-19 vaccine update: U.S. says not to join WHO-linked effort for coronavirus vaccine The White House has opted out of the Covax project, preferring a go-it-alone approach that worries public health experts when critics say move to go ... |
 | Top Russian and Chinese COVID-19 vaccines prove potential shortcomings High-profile COVID-19 vaccines developed in Russia and China share possible effectiveness limitations as they based on a common cold virus that many people have been ... |
 | China approves COVID-19 vaccine candidate for emergency use China has approved the use of Sinovac Biotech Ltd’s coronavirus vaccine candidate CoronaVac for emergency cases as part of its program to vaccinate high-risk groups ... |
Recommended
 World
World
India strikes back at terrorists with Operation Sindoor
 World
World
India sending Holy Relics of Lord Buddha to Vietnam a special gesture, has generated tremendous spiritual faith: Kiren Rijiju
 World
World
Why the India-US Sonobuoy Co-Production Agreement Matters
 World
World
Vietnam’s 50-year Reunification Celebration Garners Argentine Press’s Attention
 World
World
"Will continue offering our full support to Indian govt": US FBI Director after Pahalgam attack
 World
World
"Great Leader": JD Vance Lauds PM Modi During His India Visit
 World
World
Trump’s Tariff Pause: A Strategic Move from “The Art of the Deal”?
 World
World