Many elderly people participate in phase two human trials of Made-in-Vietnam Covid-19 vaccine
| A close look at AstraZeneca Covid-19 vaccine at cold storages in HCMC | |
| Hanoi eyes COVID-19 vaccination for both residents and non-residents | |
| Vietnam, UK should resume exchange of delegations soon |
The second stage has been carried out by the university and the Ho Chi Minh City-based Pasteur Institute since February 26. The trials have been taking place at the university in Hanoi along with the medical center of Ben Luc district in the southern province of Long An, featuring the participation of 560 volunteers aged between 12 and 75, including 80 individuals over the age of 60, according to VOV.
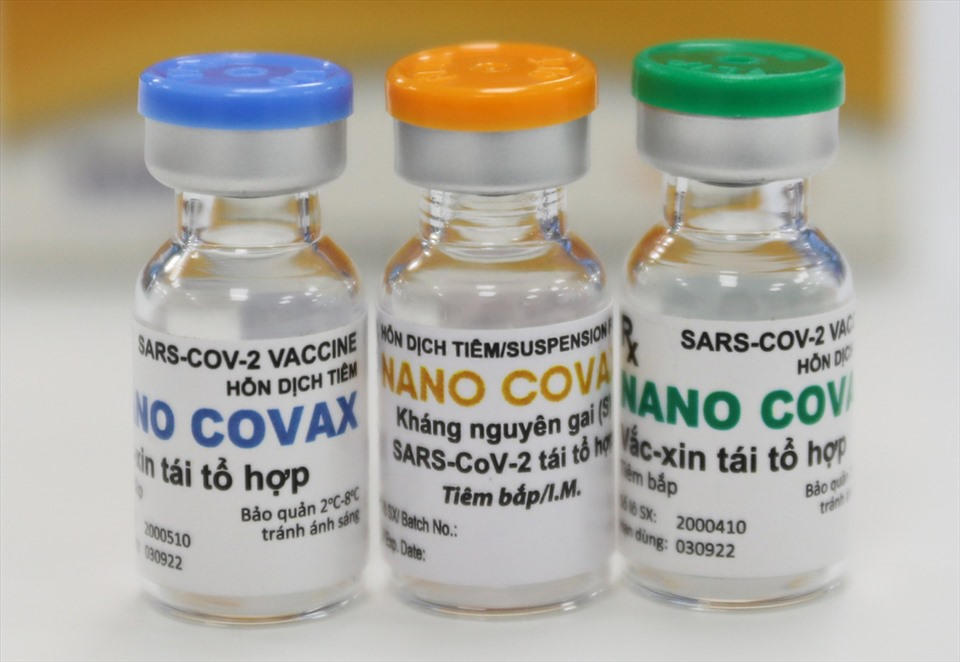 |
| Nanocovax is Vietnam’s first candidate vaccine against the novel coronavirus to reach the human trial stage. Photo: Lao dong (Labour) newspaper |
Each volunteer will receive two doses of either the vaccine or the placebo AIPO4, with an interval of 28 days between each dose. The volunteers will then be monitored for 12 months after receiving their first dose.
Volunteers who were given their initial shots on the morning of February 26 will then receive their second doses in late March.
Lieutenant General Do Quyet, director of the Military Medical University, said since the trial involves the participation of numerous volunteers suffering from underlying health conditions, competent authorities have prepared for a range of scenarios to ensure the safety of all volunteers.
Results of the trial are set to be announced in May before preparing for the third stage of trials, during which only one single shot of the vaccine will be administered to between 10,000 and 15,000 people from both domestic and foreign pandemic-hit regions, Lieut. Gen. Quyet added.
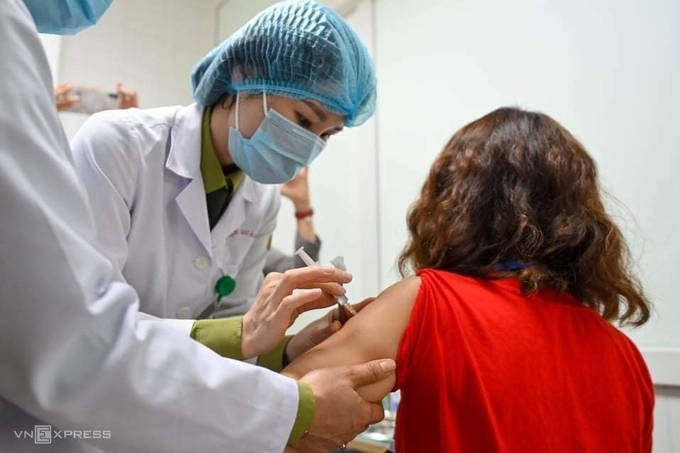 |
| First volunteer receiving Nanocovax vaccine on its second trial in Hanoi on February 26 morning (Photo: VNE) |
Originally developed by the Nanogen Pharmaceutical Biotechnology JSC and the Hanoi-based Military Medical University, Nano Covax represents the nation’s first COVID-19 vaccine which has successfully reached the human trial stage.
The initial stage of trials of the Nano Covax vaccine indicates that it is likely to be effective against the B117 variant from the UK.
Vietnam has completed 65 percent of the second phase of human trials for the Nanocovax vaccine.
Meanwhile, the country is also recruiting volunteers for the first phase of human trials of Covivac, the second homegrown Covid-19 vaccine.
Duong Huu Thai, IVAC Director on March 4 said that the Institute had completed training stages on testing procedures, examination, and volunteer recruitment for the first phase of human trials of the Covivac vaccine.
From March 5, IVAC, in coordination with the National Institute of Hygiene And Epidemiology and Hanoi Medical University would begin to receive registration from volunteers. Volunteers can enroll directly at Hanoi Medical University, by phone, email, or website, Thai added.
The first phase of human trials of the Covivac vaccine will be conducted on 120 volunteers aged 18-59. Volunteers must have no underlying health conditions and have appropriate weight and height.
Volunteers will have their health checked 9 times within 13 months since taking part in the trial. They will be inoculated with two shots, each 28 days apart. After the injection, they will remain at the clinical trial area for 24 hours for health monitoring.
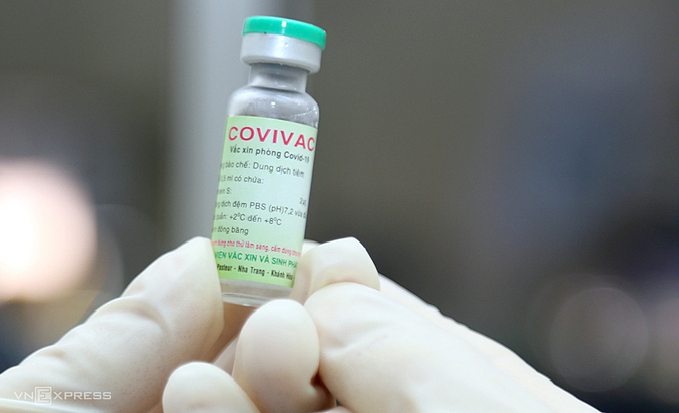 |
| A vial of Covivac, Vietnam's second Covid-19 vaccine. Photo: VnExpress |
The first jab of the Covivac vaccine in the first phase will be given to volunteers by mid-March.
The second phase of the human trial will commence in July and the final phase in November.
Thai said that the pre-clinical studies in India, the U.S., and Vietnam have shown that Covivac is effective against the new virus variants from the U.K. and South Africa. The vaccine would cost around VND60.000 ($2.60).
Scientists are currently studying its efficacy against more strains, he added.
Vietnam has two other COVID-19 candidate vaccines being developed, which are VABIOTECH by the Company for Vaccine and Biological Production No 1, and POLYVAC by the Centre for Research and Production of Vaccines and Biologicals.
The government has also speeded up foreign procurement to vaccinate some prioritized categories of people.
It has ordered 30 million doses from AstraZeneca, and the first batch of 117,600 doses arrived in HCMC last week and will be given to people working on the front lines like medical workers, contact tracers, and officials on Covid-19 prevention and control committees, Vnexpress reported.
The first jabs of AstraZeneca vaccines will be given to prioritized groups across Vietnam starting March 8, Health Minister Nguyen Thanh Long said Friday morning.
The government has said it is also stepping up negotiations with vaccine manufacturers in the U.S., Russia, and some other countries to ensure it could get a total of 150 million doses this year to cover 70 percent of its population.
 | Vietnam to administer first doses of COVID-19 vaccine next Monday The first jabs of AstraZeneca vaccines will be given to prioritized groups across Vietnam starting March 8, Health Minister Nguyen Thanh Long said Friday morning. |
 | Vietnam recruits volunteers for phase one human trials of 2nd homegrown Covid-19 vaccine The Institute of Vaccines and Medical Biologicals (IVAC) is recruiting volunteers for the first phase of human trials of Covivac, the second Made-in-Vietnam Covid-19 vaccine. |
 | European begans review of Russia’s Sputnik V vaccine, studying on it’s efficacy The European Medicines Agency (EMA) announced that it will begin to access and study the Russian coronavirus vaccine’s efficacy, Sputnik V, as the bloc looks ... |
Recommended
 Viet's Home
Viet's Home
Vietnam's Human Development Index Remains High
 Viet's Home
Viet's Home
Vietnam’s Mark on UN Day of Vesak Celebrations
 Viet's Home
Viet's Home
Art Program Spreads Message of Peace Worldwide
 Expats in Vietnam
Expats in Vietnam
Look Forward to New Developments in Vietnam - US Relations
 Viet's Home
Viet's Home
She Feeds the World: 8,000 Individuals Adopt More Sustainable Agricultural Practices
 Viet's Home
Viet's Home
Over 200 Valuable Documents Displayed at 'Mountains and Rivers Connected One Strip' Exhibition
 Viet's Home
Viet's Home
Latin American News Agency Prensa Latina Shares Story of Vietnamese Veteran’s 1,200km Journey Back to Former Battlefield
 Viet's Home
Viet's Home





