Vietnam's COVID-19 vaccine trial: Requirements for volunteers
 |
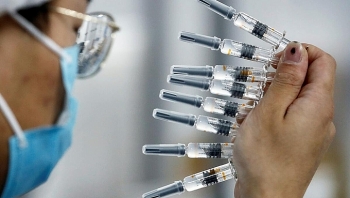
| Vietnam currently has 4 research units for the Covid-19 vaccine. (Photo: BBC) |
Vietnam currently has 4 research units for the Covid-19 vaccine, of which Nanogen has the fastest progress. Starting from December 10, the company will recruit volunteers for clinical trials. According to Mr. Ho Nhan - General Director of Nanogen Company, in the 1st phase of the clinical trial, the company will coordinate with the Military Medical Academy in recruiting 60 volunteers for injection.
The selection and physical check for all who want to become volunteers will last for 1 week, starting from December 17. In order to be selected, one must be healthy, at the age from 18 to 60, has no infectious diseases, no underlying disease, and no allergic status. Volunteers in this group will continue to be divided into 3 smaller groups to test different levels of vaccine, from 15 mg to 100 mg.
According to Nguyen Ngo Quang, Deputy Director of the Department of Science, Technology, and Training under the Ministry of Health (MoH), and leading experts in the field of vaccine research and testing, volunteers involved in the first phrase must have comprehensive understandings of the vaccine, as well as the trial process. All citizens, regardless of ethnicity or geographical conditions are welcome to enroll, except sensitive ones, such as pregnant women. The first phase will test the safety of the new vaccine.
The entire phase 1 will last for 1 month. However, because the Ministry of Health allows the implementation of knee-head research, as soon as phase 1 conducts more than 50% of the data, the clinical trial of phase 2 will be deployed.
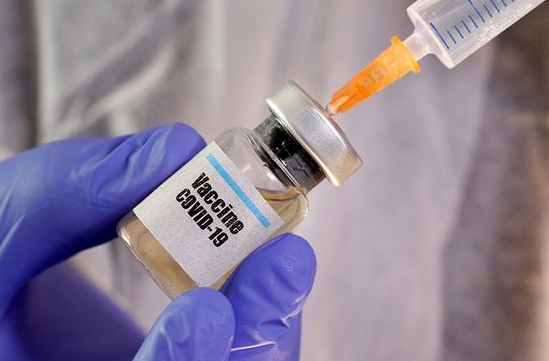 |
| A series of countries will introduce their Covid-19 vaccines into the market, including the UK, Russia, and China. (Photo: Getty) |
Phase 2 will be carried out on a scale of 600 people, for a period of 2-3 months. After that, the third phase will be continued. If all goes well, the clinical trial will be completed by April and May be vaccinated. Phase 2 and 3 will test the efficiency of the vaccine, so volunteers selected will be people who were already infected with the SAR-CoV-2 Virus, said Nguyen Ngo Quang.
According to leaders Nanogen, the company can produce 30 million doses of Covid-19 vaccine per year at a cost of about 100,000 VND ((4,3 USD)/ dose. Each person will receive 2 injections, half a month apart.
Quang said that the Ministry of Health was planning to carry on the vaccine trial in India, Bangladesh, and Indonesia to have better judgments on the efficiency of the vaccine.
Currently, in addition to Nanogen, Vietnam has 3 production and research units producing Covid-19 vaccines including IVAC, Vabiotech, and Polyvac. IVAC will begin its clinical trials in February 2021 and Vabiotech will enter the clinical trial progress in March 2021.
According to the conventional vaccine production process, it will take 7-12 years, but in the case of the Covid-19 vaccine, this process is shortened into12-14 months, due to the urgency of the situation. In December 2020 and January 2-21, a series of countries will introduce their Covid-19 vaccines into the market, including the UK, Russia, and China.
 | In video: View into Vietnam's COVID-19 vaccine manufacturing factory Nanogen's Nanocovax, a potential COVID-19 vaccine candidate in Vietnam, is entering human trials this week. With qualified researchers, state-of-the-art equipment, Nanogen's vaccine is under prompt ... |
 | Covid-19 vaccination: Many countries begin vaccinations as massive rollout starts Many countries over the world started to roll out coronavirus vaccines to the public. |
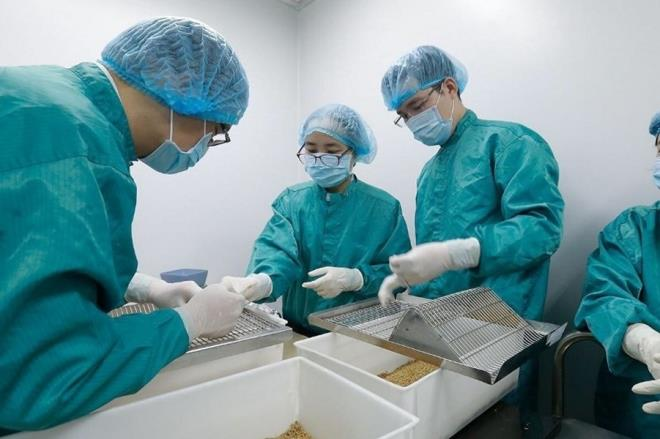 | Vietnam yet to purchase Russian Covid-19 vaccine, prepares to put indegious one on human trials The made-in-Vietnam vaccine has been confirmed to produce a good immune response against the novel coronavirus in animals and would be soon to put on ... |
Recommended
 Viet's Home
Viet's Home
Hanoi, South Africa Strengthens People-to-people Exchanges, Expands Multi-sector Cooperation
 Viet's Home
Viet's Home
Hue City to Raise Awareness on Mine Accident Prevention
 Focus
Focus
Vietnam Leaves Imprints on the World Peacekeeping Map
 Viet's Home
Viet's Home
“Global Vietnamese Singing 2025” - Connecting Hearts Longing for Homeland
 Viet's Home
Viet's Home
Vietnam’s People's Public Security Force Actively Contributes to UN Peacekeeping Operations
 Viet's Home
Viet's Home
HAUFO Enhances Competence of People-to-People Diplomacy Personnel
 Viet's Home
Viet's Home
Hands that Reserve Da Long Brocade Craft
 Viet's Home
Viet's Home