WHO: Russian COVID-19 vaccine requires rigorous safety data evaluation
It is reported by the Reuters that President Vladimir Putin on Tuesday announced that Russia had become the first country in the world to grant regulatory approval to a COVID-19 vaccine after less than two months of human testing, a move hailed by Moscow as evidence of its scientific prowess.
Commenting on President Vladimir Putin's statement on the COVID-19 vaccine, a WHO spokesman said “We are in close contact with the Russian health authorities, and discussions are going on with respect to possible pre-qualification of the vaccine”, adding that the pre-qualification of any vaccine needs rigorous review and assessment.
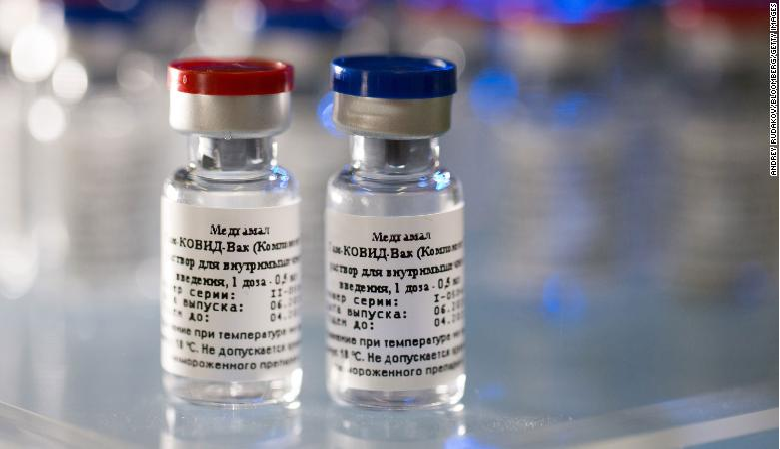 |
| Vials containing the two components of Russia's Covid-19 vaccine -- named Sputnik-V -- which has been developed by the Gamaleya Institute in Moscow. CNN |
He said as soon as the “absolutely essential clinical trial data” is available, national regulatory bodies need to be ready to review the safety and efficacy data before using it.
“We are following the progress in the development of COVID-19 vaccines, and we maintain the draft landscape of candidate vaccines, which is updated every week on our website,” added the spokesman. “WHO lists 25 candidate vaccines in clinical evaluation and 139 in pre-clinical evaluation.”
Russia’s fast-track COVID-19 vaccine triggers concerns over safety
Russia enacted a law in April which eliminated the requirement for crucial Phase 3 trials to be conducted before approval. This has allowed researchers to fast track the vaccine development process. Experts have voiced unease over Moscow's rapid approval process for the vaccine, according to CNN.
"It is unclear precisely what is actually happening with the Russian vaccine," Michael Head, Senior Research Fellow in Global Health at the University of Southampton in the UK, told the Science Medical Centre.
"It is vital that any vaccine roll-out has the confidence of the general public, and that there is good communication of the level of effectiveness and any likely side effects. At this point in time, there is no data on the Russian-led vaccine for the global health community to scrutinize."
Danny Altmann, Professor of Immunology at Imperial College London, told the Science Media Centre there were concerns about releasing a vaccine before it was fully tested.
"The bar is necessarily set very high for criteria that must be satisfied for approval after Phase 3 clinical trials," Altmann said. "The collateral damage from the release of any vaccine that was less than safe and effective would exacerbate our current problems insurmountably. I hope these criteria have been followed. We are all in this together."
 |
| An employee works with a coronavirus vaccine at the Nikolai Gamaleya National Center of Epidemiology and Microbiology in Moscow, Russia Photo: Gulf News |
Peter Hotez, a vaccine scientist at Baylor College of Medicine in Houston, Texas said “That the Russians may be skipping such measures and steps is what worries our community of vaccine scientists. If they get it wrong it could undermine the entire global enterprise”.
“This is a reckless and foolish decision. Mass vaccination with an improperly tested vaccine is unethical. Any problem with the Russian vaccination campaign would be disastrous both through its negative effects on health, but also because it would further set back the acceptance of vaccines in the population,” Francois Balloux, a geneticist at University College London, said in a statement distributed by the UK Science Media Centre.
| Developed by the Moscow-based Gamaleya Institute, the vaccine has been named Sputnik-V, a reference to the surprise 1957 launch of the world's first satellite by the Soviet Union. It has yet to go through crucial Phase 3 trials where it would be administered to thousands of people, reported CNN. The claim of victory by Putin in the global push to make an effective vaccine against Covid-19 comes amid suggestions that Russia has cut essential corners in its development. Critics say the country's push for a vaccine is partly due to political pressure from the Kremlin, which is keen to portray Russia as a global scientific force. |
 | When Covid-19 comes to an end? Although scientists are racing to find a cure for the virus, there's a chance COVID-19 will never fully go away — with or without a ... |
 | Researchers determine materials making least effective masks for Covid-19 prevention Researchers at Duke University in the US launched an experiment to determine which types of masks are quite literally useless in shielding people from Covid-19 ... |
 | Covid-19 vaccine will not be a magic bullet, mask-wearing still be critical As countries are rushing to research and produce vaccines that can put a swift end to Covid-19 pandemic, health experts still warned that vaccines are ... |
Recommended
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World