China claims that Coronavirus vaccine could be ready by April
| Volunteers offered £3,500 to be infected with coronavirus | |
| Behind the scenes: Scientists prepare for COVID-19 vaccine test | |
| Breakthrough for the COVID-19 vaccine in poultry, adaptable to humans |
 |
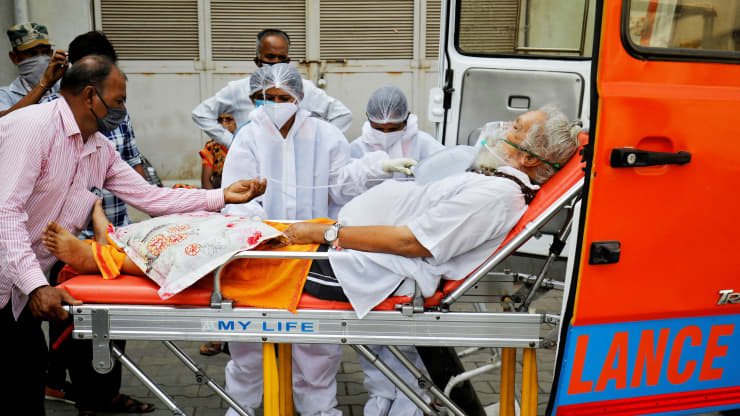
| China's National Health Commission expects the first vaccines to be in 'clinical or emergency use' next month. The picture shows China's President Xi learning about the progress on the vaccine development at the Academy of Military Medical Sciences in Beijing on March 2 (Photo source: dailymail.co.uk) |
According to nypost.com, Chinese officials say they’ll have a coronavirus vaccine ready next month for emergency situations and clinical trials.
Eight institutes in the country are working on five approaches to inoculations in an effort to combat COVID-19, according to the South China Morning Post. The contagious illness has sickened more than 118,000 people and killed at least 4,200 worldwide, mostly in mainland China, as of Tuesday afternoon.
“According to our estimates, we are hopeful that in April some of the vaccines will enter clinical research or be of use in emergency situations,” Zheng Zhongwei, director of the National Health Commission’s Science and Technology Development Center, said Friday.
While it would take at least 12 to 18 months to ensure the vaccines are safe for the general public, under Chinese law, they can be deployed earlier for urgent use in a major public health emergency, as long as the benefits outweigh the risks.
Zheng waved off concerns about the safety of the vaccines, saying they were being developed in accordance with “scientific and standardized technical requirements,” The Australian reported.
And on dailymail.co.uk's news, Mr Zheng added: 'The novel coronavirus is a new virus. We need a process to explore and understand it.
'The same applies to the development of vaccines. We need to solve problems gradually as we continue to explore and deepen [the research].'
Mr Zheng made the comments at a press conference today held by the Joint Prevention and Control Mechanism of the State Council of China.
The official said a special team had been set up to take the charge of the development of vaccines for the coronavirus.
In the US, Massachusetts-based biotechnology company Moderna Inc. shipped its vaccine to the National Institute of Allergy and Infectious Diseases for testing late last month. Initial results could be released by July or August.
 | Breakthrough for the COVID-19 vaccine in poultry, adaptable to humans Researchers at the Miguel Institute, supported by the Ministry of Science and Technology, explain the research at a press conference. They claim the vaccine’s efficacy ... |
 | Two influenza vaccines successfully produced in Vietnam Two influenza vaccines, IVACFLU-S and IVACFLY, which have been successfully produced in Vietnam, were introduced at a workshop held by the Institute of Vaccines and ... |
 | Instalment payment vaccine packages launched High-quality vaccines are available to people on low income with a zero percent interest rate on instalment payments. |
Recommended
 World
World
India strikes back at terrorists with Operation Sindoor
 World
World
India sending Holy Relics of Lord Buddha to Vietnam a special gesture, has generated tremendous spiritual faith: Kiren Rijiju
 World
World
Why the India-US Sonobuoy Co-Production Agreement Matters
 World
World
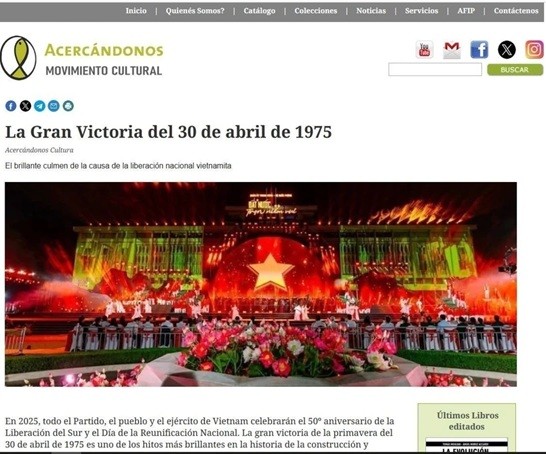
Vietnam’s 50-year Reunification Celebration Garners Argentine Press’s Attention
 World
World
"Will continue offering our full support to Indian govt": US FBI Director after Pahalgam attack
 World
World
"Great Leader": JD Vance Lauds PM Modi During His India Visit
 World
World
Trump’s Tariff Pause: A Strategic Move from “The Art of the Deal”?
 World
World