Coronavirus vaccine updates: Oxford University vaccine trial halted after participant falls ill
 |
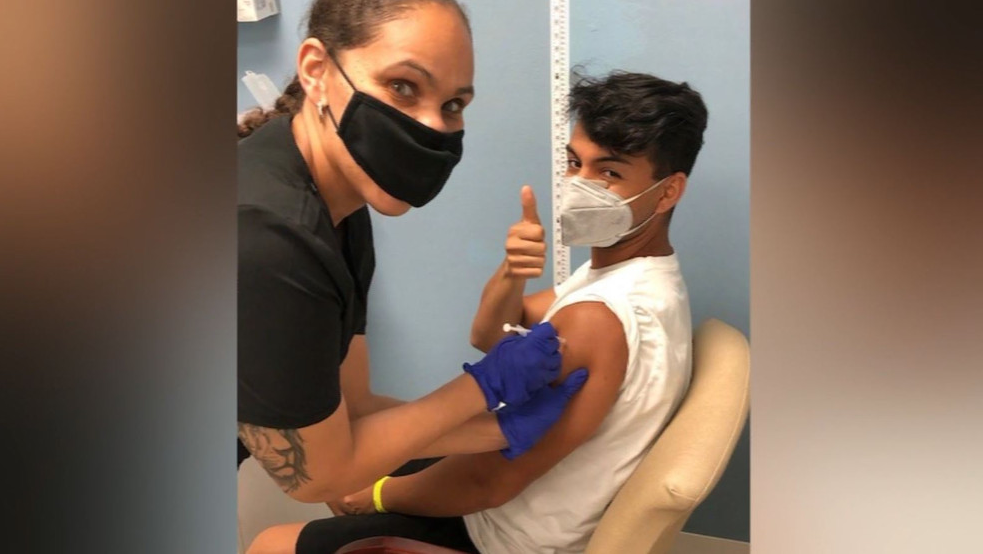
| A seasonal influenza vaccine from a nurse at a local pharmacy clinic in Johannesburg, South Africa on Friday, April 24, 2020. Photo: AP |
A large trial of the candidate coronavirus vaccine developed by Oxford University has been put on hold in the United States after a volunteer suffered a possible serious adverse reaction, it was reported last night.
The late-stage COVID-19 candidate vaccine trials run by AstraZeneca have been paused after a participant fell ill.
The company is investigating whether the vaccine recipient's "potentially unexplained” illness is a result of receiving the shot.
In a statement, AstraZeneca said the “standard review process triggered a pause to vaccination to allow review of safety data" without revealing what the potential side effect of the vaccine was.
A spokeswoman for Astrazeneca, the British drugmaker that is partnering with Oxford University, told The Times in a statement: “As part of the ongoing randomised, controlled global trials of the Oxford coronavirus vaccine, our standard review process was triggered and we voluntarily paused vaccination to allow review of safety data by an independent committee".
 |
| The pause was “a routine action” while the volunteer’s illness was investigated. Photo: thetimes.co.uk |
AstraZeneca is trialling the Oxford University vaccine which was developed using a chimpanzee adenovirus. The adenovirus was then genetically modified so that it cannot grow in humans.
Typically, the virus causes the common cold in chimpanzees.
The news about the trial's pause was first reported by health news site STAT, which reported the participant was located in the United Kingdom.
AstraZeneca had been recruiting 30,000 participants in the United States for late-stage trials of the vaccine and is also testing it in thousands of people in the United Kingdom as well as smaller studies in South Africa and Brazil.
Ellie Murray, an epidemiologist at Boston University, wrote on Twitter that determining whether the vaccine produced the illness is why the trial is being stopped. She explained that this was the point of a Phase III trial, stating: "This is the system working.", according to the euronews.
The news of the pause came the same day AstraZeneca, along with eight other CEOs, made a pledge to "make the safety and well-being of vaccinated individuals the top priority in development of the first COVID-19 vaccines."
What does it mean if one trial patient got sick?
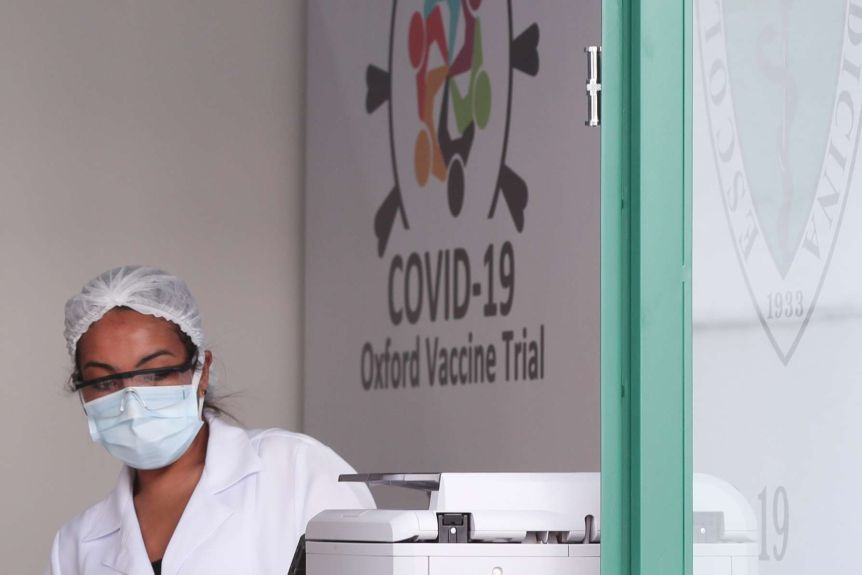 |
| The Oxford/AstraZeneca coronavirus vaccine trials are also being conducted in Sao Paulo, Brazil. Photto: Reuters |
There is a lot still to figure out.
Crucially, we don't even know yet whether the person who fell ill received the fledgling coronavirus vaccine, or the control meningitis vaccine — just that AstraZeneca's "standard review process triggered a pause in vaccination".
That's partly by design. So as not to bias the results, trials like this are blinded; nobody directly involved knows which patients are receiving which vaccine.
![Vinicius Molla, a haematologist and volunteer in the clinical trial of the Oxford COVID-19 vaccine, examines a patient in a consulting room in Sao Paulo, Brazil [File: Amanda Perobelli/Reuters] 5416 va3](https://vietnamtimes.org.vn/stores/news_dataimages/thuyhangvnt/092020/09/15/in_article/5416_va3.png?rt=20200909155417) |
| The clinical trial of the Oxford COVID-19 vaccine, examineing a patient in a consulting room in Sao Paulo, Brazil. Photto: Reuters |
What happens next is down to a separate expert group known as a Data Safety Monitoring Board (DSMB), who will examine the patient's case in detail, including — if necessary — which vaccine they received.
"Then the DSMB is asked to determine whether or not a 'pause' should lead to a 'stop', or whether the pause can be lifted and the study continue," Professor Nolan explains.
Even if the sick patient did receive the trial vaccine, their illness might still be unrelated, said the abc.
In the words of AstraZeneca's statement: "In large trials illnesses will happen by chance but must be independently reviewed to check this carefully."
Or as ABC health journalist Norman Swan put it: "Somebody's going to get a heart attack, somebody's going to get pneumonia, somebody's going to break their leg.
"You've got to work out whether this is the play of chance, or whether it's feasibly attributable to the vaccine."
 | Chinese Covid-19 vaccine’s price ten times higher than Russian’s According to the head of a major state-owned Chinese pharmaceutical company Sinopharm, its coronavirus vaccine may cost some hundred yuan per shot and around 1,000 ... |
 | Vaccine production: a billion-dollar industry According to estimates of research firm Alliance Bernstein (headquarter in the US), the global vaccine production industry is worth $ 52 billion per year. In ... |
 | Top US expert Fauci has doubts about Russian coronavirus vaccine Concocting a vaccine is not the same thing as proving a vaccine is safe and effective, said Anthony Fauci, Director of the US National Institute ... |
Recommended
 World
World
India strikes back at terrorists with Operation Sindoor
 World
World
India sending Holy Relics of Lord Buddha to Vietnam a special gesture, has generated tremendous spiritual faith: Kiren Rijiju
 World
World
Why the India-US Sonobuoy Co-Production Agreement Matters
 World
World
Vietnam’s 50-year Reunification Celebration Garners Argentine Press’s Attention
 World
World
"Will continue offering our full support to Indian govt": US FBI Director after Pahalgam attack
 World
World
"Great Leader": JD Vance Lauds PM Modi During His India Visit
 World
World
Trump’s Tariff Pause: A Strategic Move from “The Art of the Deal”?
 World
World