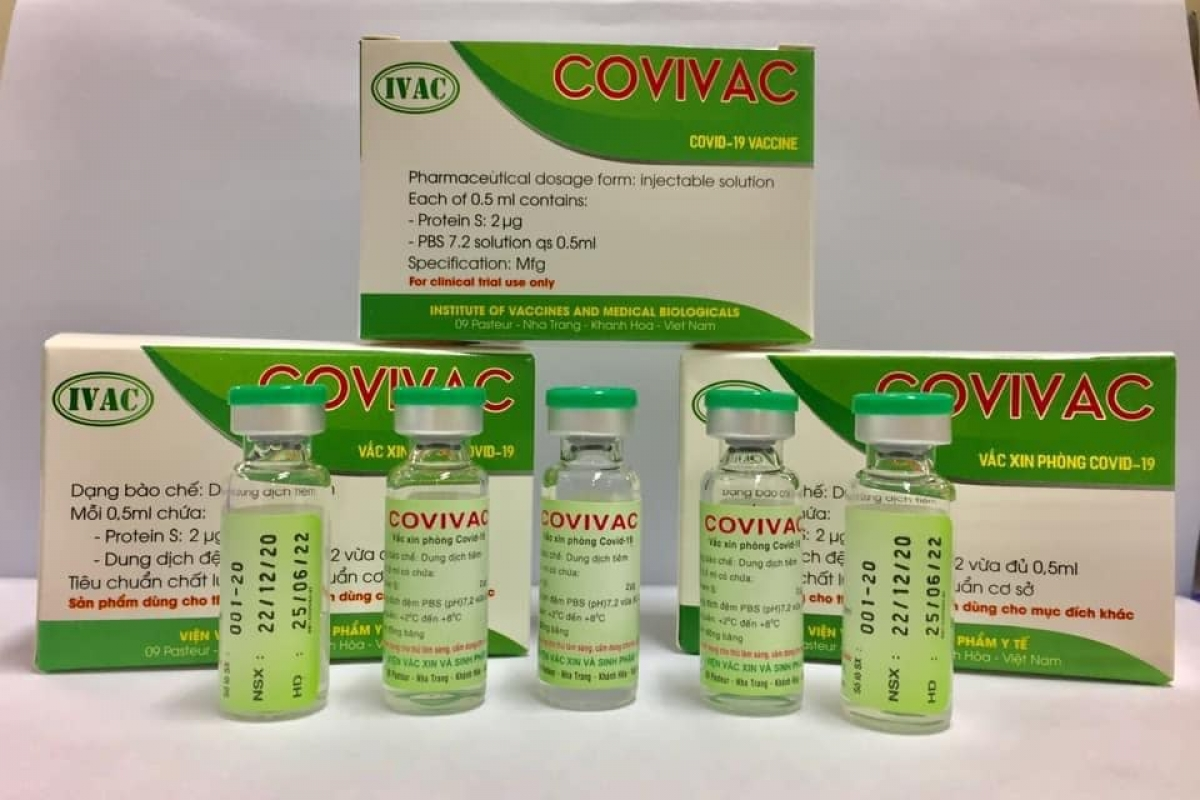
Second Vietnam-produced COVID-19 vaccine to enter human trials
COVIVAC has been sent to India and the US for trials on mice and trial results have showed a promising level of safety and efficacy, said Duong Huu Thai, head of the IVAC. Accordingly, IVAC has proposed the Ministry to allow the human trials.
It is scheduled that during the first phase on January 21 and 22, 125 volunteers will receive different doses. During the second phase, the vaccine will be injected on 250 volunteers.
Volunteers will begin trials this month at the Hanoi Medical University in the capital.
“The vaccine was proved safe to the animals and reported strong immune response in the animals,” Head of IVAC Duong Huu Thai said.
Head of IVAC Thai added that the human trial will include three phases. However, the third phase will heavily depend on the first and the second phase’s results.
Thai said that the vaccine had been studied and developed since May.
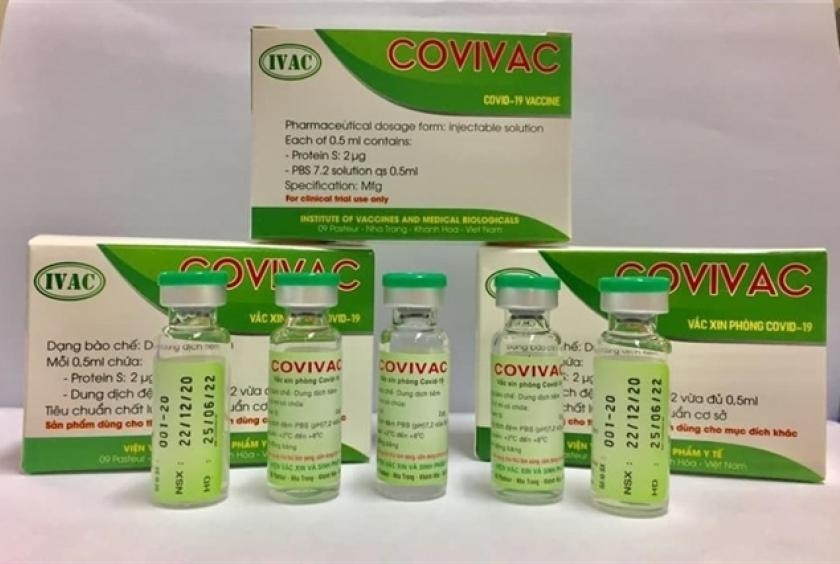 |
Second Vietnam-produced COVID-19 vaccine COVIVAC, is manufactured by the Institute of Vaccines and Medical Biologicals |
How was COVIVAC developed?
COVIVAC was developed using IVAC derived from the Newcastle disease virus (NDV) LaSota strain handed over by the U.S. back in May last year, routinely used as a live vaccine globally. The NDV in general is a virus that causes a deadly infection in many kinds of birds and flu-like symptoms in humans.
IVAC decided to develop a vaccine using primary French chicken embryo cultures, a technique the institute had used in the past to successfully produce seasonal flu and H5N1 bird flu vaccines.
The NDV-Lasota vaccine strain was injected into the egg for the virus to grow before the fluid was drained for further refining and filtering. Inactivation of the virus, though with all characteristics in situ, finally produced Covivac.
The institute subsequently requested the Health Ministry's permission to commence human trials at the end of this month after completing tests on mice, rabbits and monkeys.
Currently, Vietnam has four COVID-19 vaccines under development by Nanogen Pharmaceutical Biotechnology JSC, the Institute of Vaccines and Medical Biologicals (IVAC), Vaccine and Biological Production Company No. 1 (Vabiotech) and the Center for Research and Production of Vaccines and Biologicals (Polyvac).
On December 17, 2020, the first Vietnam-made COVID-19 vaccine NANOCOVAX developed by Nanogen Pharmaceutical Biotechnology JSC entered human trials with volunteers receiving the first doses at the Military Medical University in Hanoi.
The volunteers remain in a healthy condition after receiving two doses each.
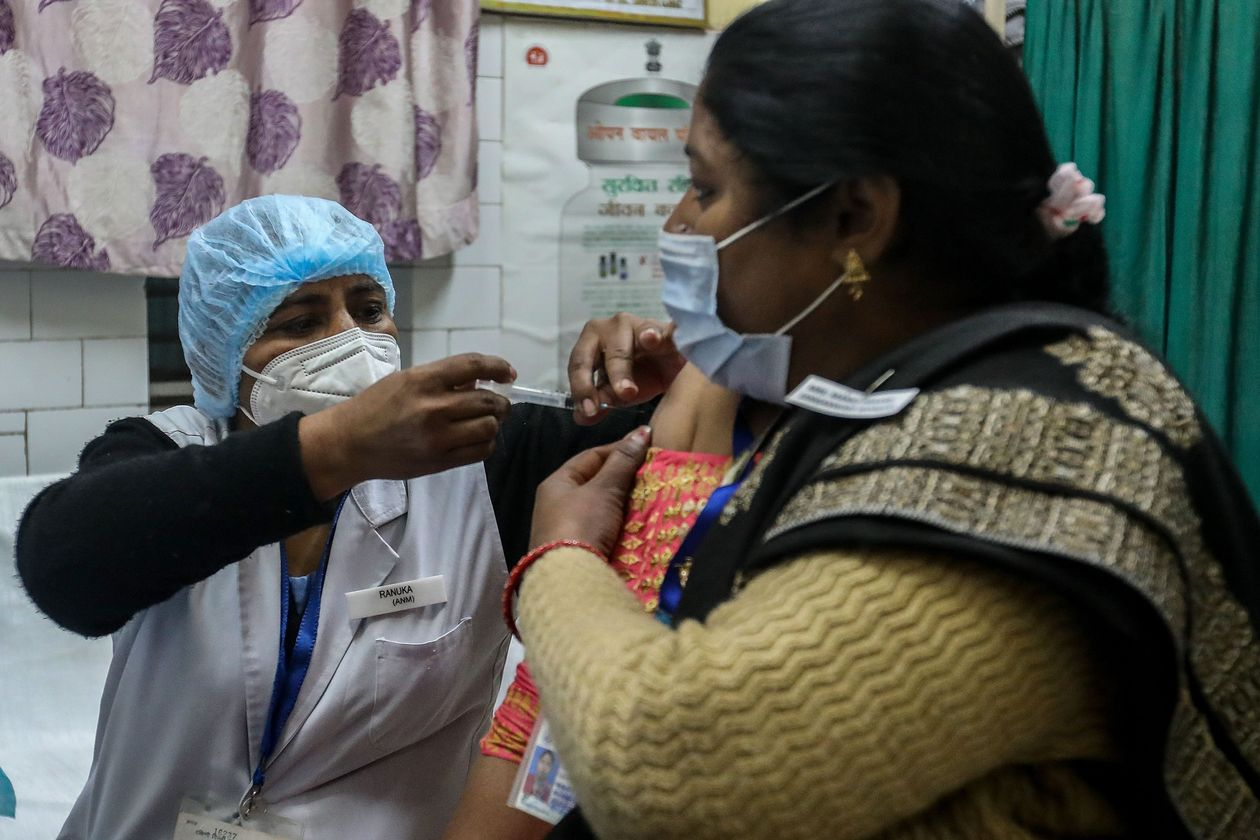 | India follows UK to grant emergency approval for AstraZeneca/Oxford Covid-19 vaccine India has followed the U.K. and granted emergency approval for the coronavirus vaccine developed by AstraZeneca Plc and the University of Oxford, the first step ... |
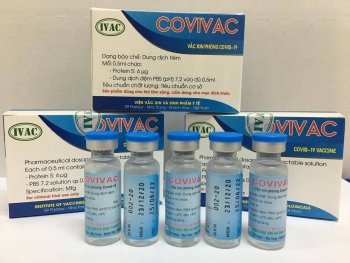 | Vietnam's second COVID-19 vaccine set to enter human trials this month IVAC's Covivac COVID-19 vaccine will enter human trials in January after safety test assessments in animals, becoming the second vaccine in Vietnam to granted such ... |
 | Vietnamese PM’s message on International Day of Epidemic Preparedness The first ever International Day of Epidemic Preparedness, being held on 27 December 2020, was called for by the United Nations General Assembly to advocate ... |
Recommended
 Viet's Home
Viet's Home
Hanoi, South Africa Strengthens People-to-people Exchanges, Expands Multi-sector Cooperation
 Viet's Home
Viet's Home
Hue City to Raise Awareness on Mine Accident Prevention
 Focus
Focus
Vietnam Leaves Imprints on the World Peacekeeping Map
 Viet's Home
Viet's Home
“Global Vietnamese Singing 2025” - Connecting Hearts Longing for Homeland
 Viet's Home
Viet's Home
Vietnam’s People's Public Security Force Actively Contributes to UN Peacekeeping Operations
 Viet's Home
Viet's Home
HAUFO Enhances Competence of People-to-People Diplomacy Personnel
 Viet's Home
Viet's Home
Hands that Reserve Da Long Brocade Craft
 Viet's Home
Viet's Home