Advantages of UK’s Oxford – AstraZeneca vaccine that Vietnam has purchased
 |
| Oxford – AstraZeneca vaccine (Photo: Reuters) |
Deputy Minister of Health Truong Quoc Cuong on January 4 confirmed that Vietnam has signed with BioPharmaceutical Company AstraZeneca for 30 million doses of its Covid-19 vaccine. The shipment would guarantee enough vaccinations for at least 15 million Vietnamese people.
Oxford – AstraZeneca vaccine is reasonably priced at US$3-4 per dose, while Pfizer-BioNTech and Moderna vaccine is respectively charged at US$20 and US$30 per dose.
On top of that, the vaccine is easily delivered given the fact that it only requires storage temperatures from 2-8 degrees Celcius and can last for 6 months inside the normal medical refrigerator.
At the other end of the spectrum, the Pfizer-BioNTech vaccine must be stored and transported at minus 70 degrees Celcius and kept in the normal medical refrigerator at 2-8 degrees C for up to only 5 days.
How does the AstraZeneca-Oxford vaccine work?
The vaccine – called ChAdOx1 nCoV-19 – uses a harmless, weakened version of a common virus that causes a cold in chimpanzees, according to the Irish Examiner. The virus is genetically modified so that it is impossible for it to grow in humans.
Scientists have transferred the genetic instructions for coronavirus’s specific “spike protein” – which it needs to invade cells – to the vaccine.
When the vaccine enters cells inside the body, it uses this genetic code to produce the surface spike protein of the coronavirus.
This induces an immune response, priming the immune system to attack coronavirus if it infects the body.
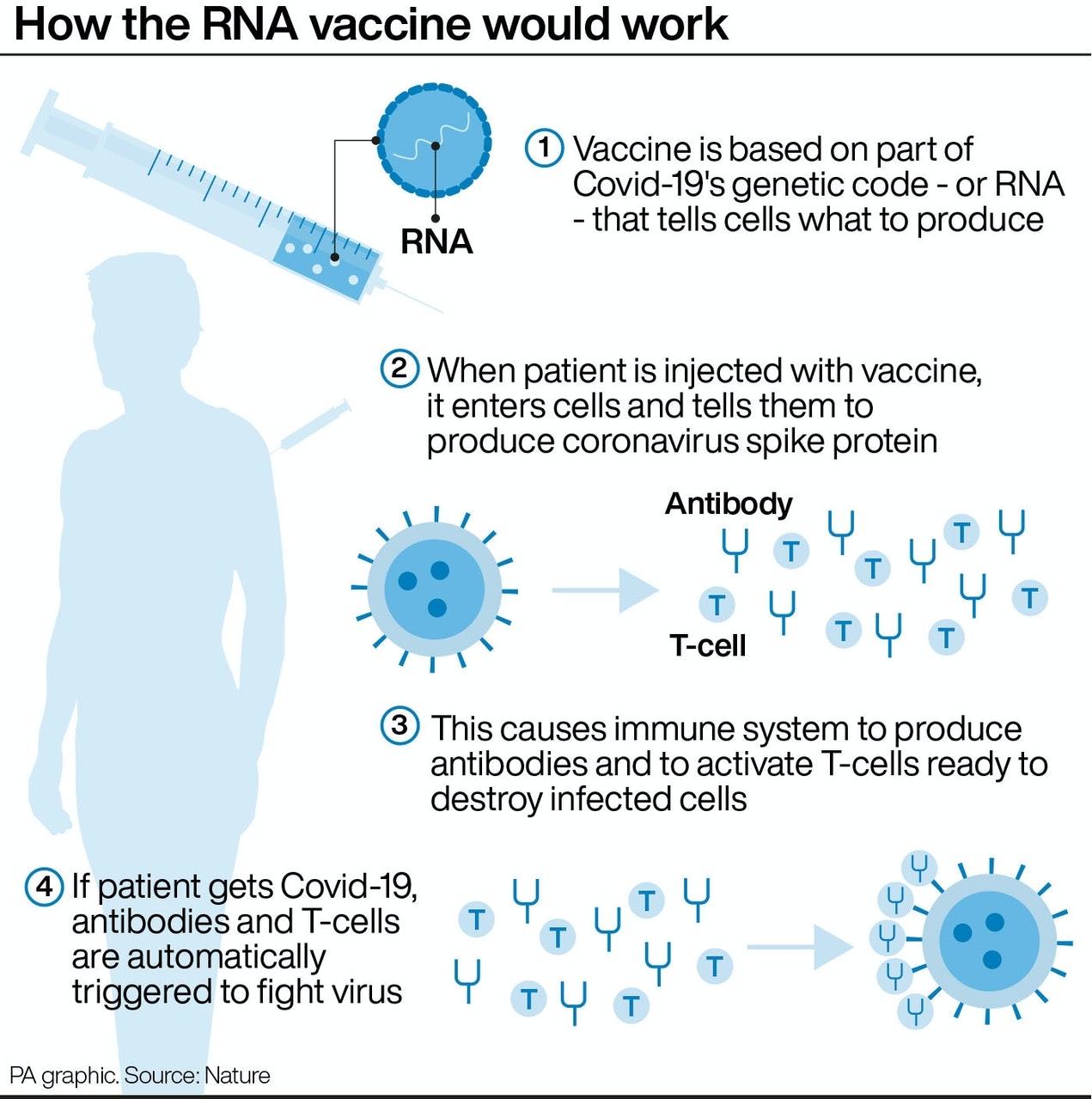 |
| (Graphic: Nature) |
How effective is it?
Phase 3 trial data showed the jab was 70.4% effective on average across two different dose regimes and possibly up to 90% when one-half dose is given followed by a further full dose.
The MHRA has recommended over 18s should receive two doses to be administered with an interval of between four and 12 weeks.
 |
| Covivac is set to enter human trial this month (Photo: VOV) |
| Meanwhile, Vietnam is having four locally-made Covid-19 vaccine candidates at hands, with the NanoCovax vaccine is the first to enter human trials, expected to enter mass production in May 2021. The second potential "life-saver" named Covivac is set to go into the first phase of human trials this January. The remaining two are being evaluated on animals. With 7 new imported Covid-19 patients, the national count of Covid-19 cases is raised to 1,504, including 693 locally transmitted cases. The number of recovered patients is now 1,339 while fatalities remain at 35. Among patients still under treatment, nine have tested negative for SARS-CoV-2 once, six twice and five thrice. A total of 19,286 people who had close contact with Covid-19 patients or returned from pandemic-hit areas are being quarantined nationwide. |
 | India follows UK to grant emergency approval for AstraZeneca/Oxford Covid-19 vaccine India has followed the U.K. and granted emergency approval for the coronavirus vaccine developed by AstraZeneca Plc and the University of Oxford, the first step ... |
 | Vietnam's second COVID-19 vaccine set to enter human trials this month IVAC's Covivac COVID-19 vaccine will enter human trials in January after safety test assessments in animals, becoming the second vaccine in Vietnam to granted such ... |
 | World breaking news today (January 1): WHO grants emergency use approval for Pfizer Covid-19 vaccine World breaking news today (January 1): WHO grants emergency use approval for Pfizer Covid-19 vaccine. Meanwhile, Trump and Melania abruptly ditched Year-end party. |
Recommended
 National
National
Vietnam News Today (May 31): Vietnam Strongly Supports Laos’s National Development
 National
National
Vietnam News Today (May 30): Vietnam, Venezuela Reinforce Ties Through People-to-people Diplomacy
 National
National
Vietnam News Today (May 29): Vietnam and Hungary to Expand Cooperation into New Areas
 National
National
Vietnam News Today (May 28): Vietnam and China Discuss Strategic Cooperation Orientations
Popular article
 National
National
Vietnam News Today (May 27): Vietnam Treasures Multifaceted Collaboration with France
 National
National
Vietnam Commits to Building an Inclusive, Sustainable and Cohesive ASEAN
 National
National
Vietnam Proposes Vision for Responsible Digital Journalism Cooperation
 National
National