Vietnam in talks with UK to buy 30 million doses of Covid-19 vaccine
The information was confirmed by Deputy Health Minister Truong Quoc Cuong at the press conference of the Government Office on the morning of January 4.
The Ministry of Health is also negotiating to purchase vaccines from the US (Pfizer), Russia (Sputnik V), and China. The negotiating parties are required to sign an information confidentiality memorandum, with only some of the contents being unveiled.
"We’re closest to an agreement with AstraZeneca to guarantee the supply of 30 million doses of vaccine between the first and fourth quarters of 2021," Deputy Minister Cuong said.
The vaccine will be administered by two doses per person, VNExpress reported.
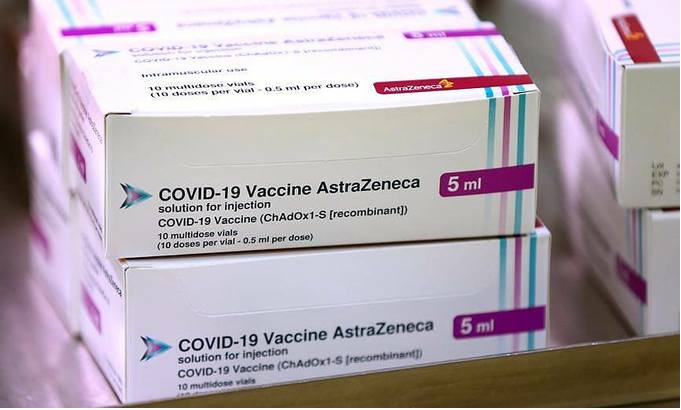 |
| The Oxford University/AstraZeneca Covid-19 vaccine boxes at the Princess Royal Hospital in Haywards Heath, West Sussex, Britain, January 2, 2021. Photo by Gareth Fuller via Reuters. |
The AstraZeneca and Oxford University Covid-19 vaccine is cheaper than some others and can be stored at fridge temperature, which makes it easier to transport and use, particularly in developing countries.
The vaccine developed by AstraZeneca costs $2-3 per dose, The Guardian reported.
Some of the vaccines in question have proven from 65 percent to 94.5 percent effective during clinical trials, Cuong said.
He also revealed Vietnam would be eligible to buy vaccines from the World Health Organization’s Covax program to cover 16 percent, or 15.6 million of its nearly 98 million population, at "the cheapest price."
The Covax facility is a global mechanism in the development, manufacturing, and procurement of Covid-19 vaccine candidates, facilitating and supporting member countries to access vaccines as they become available. Covax aims to provide two billion doses by the end of 2021, which is to cover 20 percent of the population of member countries, including Vietnam.
Nanocovax, developed by Nanogen Pharmaceutical Biotechnology JSC, entered human trials on Dec. 17 with 60 volunteers signing up for the first phase.
Researchers said they might kick off the second phase earlier right after the first phase yields 50 percent effective. Thus, according to Do Minh Si, Development Research Director of Nanogen, the clinical trials might end at the end of 2021.
 |
| Made-in-Vietnam Nanocovax vaccine Photo: VNE |
Nanocovax is priced at VND120,000 ($5.17) per dose.
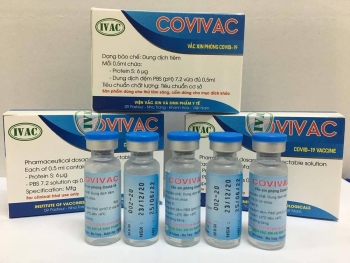
Vietnam's second COVID-19 vaccine, IVAC's Covivac COVID-19 vaccine is set to enter human trials in January after safety test assessments in animals, becoming the second vaccine in Vietnam to granted such action.
The Covivac vaccine is the research work of the Institute of Vaccines and Medical Biologicals (IVAC) under the Ministry of Health (MOH). It had earlier yielded safe results and strong immunity responses on mice, rabbits, etc. Given promising results on animals, the vaccine is granted to enter human trials starting January, two months earlier than expected.
 |
| Covivac will become the second COVID-19 vaccine made in Vietnam to enter human trials Photo: VOV |
As reported by VOV, IVAC will cooperate with the National Institute of Hygiene and Epidemiology and Hanoi Medical University to trial the vaccine on 125 volunteers aged 18-59. Those receiving IVAC’s trial jabs must be healthy, having no underlying disease, and satisfying several other specific criteria.
The first batch of volunteers will be divided into different groups who receive different doses. Following stages will be conducted if the human trials go smoothly.
According to Dr. Duong Huu Thai, Director of IVAC, the Institue has been studying Covivac since last May, aiming at successfully producing the vaccine and completing three human trials within 18 months. The first phase of the trails is scheduled to finish in April.
“If all three trial phases yield good results, Vietnam might have the vaccine in late 2021”, Thai said. “IVAC has an edge in the production process thanks to its available infrastructure, technology, and decade-long experience in producing flu vaccines”.
Vietnam currently has four Covid-19 vaccines under development, the other three by the Institute of Vaccines and Medical Biologicals (IVAC), Vaccine and Biological Production Company No. 1 (Vabiotech), and the Center for Research and Production of Vaccines and Biologicals (Polyvac).
Globally, 11 vaccines have entered the third phase of clinical trials to date.
| Vietnam has recorded 1,494 COVID-19 cases so far. As many as 1,339 patients have fully recovered from the disease while the number of related deaths is still kept at 35. Among the patients undergoing treatment across the country, nine have tested negative for the virus once, six others twice, and five thrice. 18,743 people who had close contact with COVID-19 patients or entered Vietnam from pandemic-hit regions are currently quarantined nationwide, including 150 in hospitals, 17,008 in state-designated establishments, and 1,575 at their residences. |
 | Vietnamese PM’s message on International Day of Epidemic Preparedness The first ever International Day of Epidemic Preparedness, being held on 27 December 2020, was called for by the United Nations General Assembly to advocate ... |
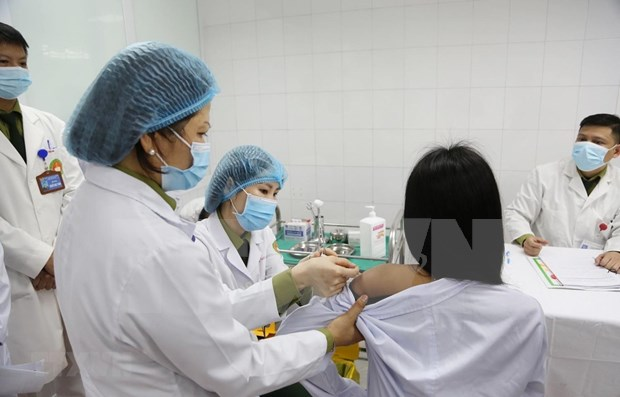 | Three volunteers injected 50mcg dose of Made-in-Vietnam Covid-19 vaccine On December 26 at the Hanoi-based Military Medical Academy, three local volunteers received a 50mcg dose of the Made-in-Vietnam Covid-19 vaccine Nano Covax. |
 | In Christmas message, Pope Francis calls for COVID-19 vaccines to be shared On a Christmas like no other, Pope Francis prayed for people who could not be with their families because of the COVID-19 pandemic, and called ... |
Recommended
 Friendship
Friendship
Dr. Vu Hoai Chuong Receives Hungary's Knight Cross Order
 Friendship
Friendship
Promoting Vietnam - Japan Economic Cooperation
 Friendship
Friendship
VUFO Attends Fourth Dialogue on Exchange and Mutual Learning among Civilizations
 Friendship
Friendship
COPI (US) Provides Free Medical Check-Ups for Nearly 1,000 People in Quang Nam
 Focus
Focus
Strengthen Solidarity and Friendship Between Vietnam and Venezuela
 Friendship
Friendship
VUFO Supports Initiatives to Enhance People-to-people Exchanges between Vietnam and the Philippines
 Friendship
Friendship
Quang Ngai Recognizes Cuban Health Experts' Contributions to Mother and Child Care
 Friendship
Friendship