Three volunteers injected 50mcg dose of Made-in-Vietnam Covid-19 vaccine
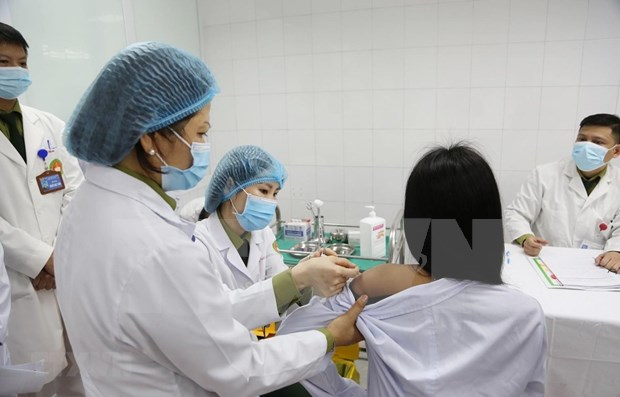 |
| One of the three first volunteers receiving first shots of 50mcg dose of COVID-19 vaccine (Photo: VNA) |
The trio is among a group of 20 volunteers who are scheduled to receive the 50mcg dose as part of the ongoing human clinical trial campaign which is being carried out by Nano Covax producer, Nanogen Pharmaceutical Biotechnology JSC, and the Military Medical Academy, VOV reported.
The three volunteers will remain under close scrutiny at the Academy over the course of the subsequent 72 hours. Providing that they stay healthy, the group’s remaining 17 volunteers will then receive a similar dose.
The first group of 20 volunteers received a 25mcg dose of Nano Covax on December 17 and have so far shown no signs of any negative side effects, with the exception of a mild temperature of less than 37.8 degrees Celsius.
 |
| |
“Based on the encouraging results, the Ministry of Health has permitted the Military Medical Academy to continue with the 50mcg injection on a further 20 volunteers,” said Major General, Prof. Dr. Hoang Van Luong, deputy director of the Military Medical Academy.
According to Gen. Luong, an additional group of 20 volunteers will subsequently be given a 75mcg dose of Nano Covax over the coming days in an effort to test the vaccine’s safety.
The Military Medical Academy is now recruiting volunteers to take part in the second and third phases which are scheduled to start in March and August 2021, respectively.
The human trials of Nanocovax will go through three phases. In the first phase, 60 volunteers are divided into three groups to receive three doses of 25 mcg, 50 mcg, and 75 mcg of the vaccine. In the second phase, the vaccine will be injected into 400-600 volunteers aged from 12 to 75 to determine the optimal dose. The third phase will be carried out on at least 1,500 to 3,000 volunteers and even up to 10,000 to 30,000 volunteers. This phase aims to evaluate the safety, immunity, and efficacy of the vaccine.
| Vietnam currently has four Covid-19 vaccines under development, the other three by the Institute of Vaccines and Medical Biologicals (IVAC), Vaccine and Biological Production Company No. 1 (Vabiotech), and the Center for Research and Production of Vaccines and Biologicals (Polyvac). The vaccines developed by IVAC and Vabiotech are expected to enter human trials in early 2021. Vietnam also has plans to import Covid-19 vaccines. Globally, 11 vaccines have entered phase three clinical trials to date. |
| On December 26, a man in the Mekong Delta's Vinh Long Province was confirmed to test positive for the novel coronavirus after illegally entering Vietnam from Cambodia, raising the country's infection tally to 1,440. Of Vietnam's patients, 99 are still active, and there have been 35 deaths. No community transmission has been recorded nationally in nearly a month. |
 | Thousands of volunteers to be injected with Vietnam's first Covid-19 vaccine Leaders of the Ministry of Health said that 10,000 to 30,000 volunteers are expected to participate in the third phase of human trials of Nanocovax, ... |
 | All three Vietnamese volunteers injected with Covid-19 vaccines healthy After three days vaccinated with the Nano Covax vaccine, three Vietnamese volunteers are in stable health conditions with no unusual reactions. |
 | COVID-19 Updates (Dec. 21): Deputy PM visits first COVID-19 vaccine trial volunteers The first three volunteers receiving Nano Covax - the Vietnamese-made anti-COVID-19 vaccine - are in good health conditions 72 hours after injection |
Recommended
 Viet's Home
Viet's Home
Hanoi, South Africa Strengthens People-to-people Exchanges, Expands Multi-sector Cooperation
 Viet's Home
Viet's Home
Hue City to Raise Awareness on Mine Accident Prevention
 Focus
Focus
Vietnam Leaves Imprints on the World Peacekeeping Map
 Viet's Home
Viet's Home
“Global Vietnamese Singing 2025” - Connecting Hearts Longing for Homeland
 Viet's Home
Viet's Home
Vietnam’s People's Public Security Force Actively Contributes to UN Peacekeeping Operations
 Viet's Home
Viet's Home
HAUFO Enhances Competence of People-to-People Diplomacy Personnel
 Viet's Home
Viet's Home
Hands that Reserve Da Long Brocade Craft
 Viet's Home
Viet's Home