Covid-19 vaccine update: Controversial Russia’s ‘Sputnik V’ and its availability
 |
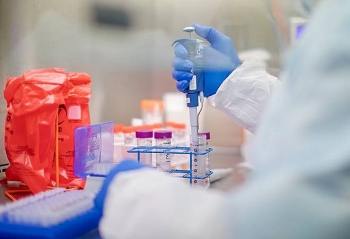
| Russian President Vladimir Putin claims the vaccine offers “sustainable immunity” against Covid-19.(AFP) |
Ever since Russia dropped the bombshell announcement of giving a regulatory approval for the “Sputnik V” vaccine for Covid-19on Tuesday, the development has been met by scepticism. One of the biggest concerns is that the approval comes before the completion of human trials. Russia was yet to start a larger trial involving thousands of participants, commonly known as a Phase III trial, which is normally considered an essential precursor to a regulatory approval.
Russian President Vladimir Putin claims the vaccine offers “sustainable immunity” against Covid-19. He said one of his daughters had received the inoculation and felt better, said the hindustantimes.
The vaccine was developed by the Moscow-based Gamaleya Institute, using funding from the Russian Direct Investment Fund (RDIF). The vaccine is named Sputnik V -- a reference to the 1957 Soviet Union satellite.
Scientists conducted months of human trials but are yet to publish data and did not begin the crucial Phase 3 stage, which usually precedes approval, before the announcement on Tuesday.
 |
| Russia just approved a coronavirus vaccine for use in tens of thousands of people, though it has not been thoroughly tested for effectiveness, according to news reports. Cited the livescience. |
On Wednesday it was announced that a Phase 3 trial involving more than 2,000 people in Russia and several Middle Eastern and Latin American countries had begun. Typically this stage of trial involves testing on tens of thousands of people.
Dr. Scott Gottlieb, former commissioner of the US Food and Drug Administration (FDA), said Tuesday that the number of people the vaccine had been tested on so far was the equivalent of a Phase 1 trial, which typically involves a small group and studies the safety of the vaccine, as per CNN.
Experts say the lack of published data on Russia’s vaccine have left scientists, health authorities and the public in the dark. There exists little clarity about how the vaccine is made and details on safety, immune response and whether it can prevent Covid-19 infection, news agency Reuters reported.
 |
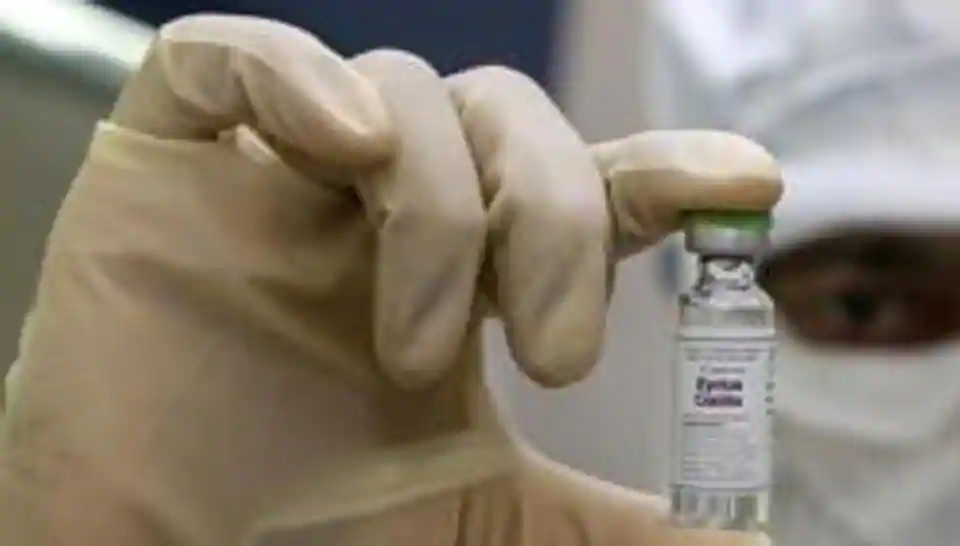
| Russian Healthcare ministry (Minzdrav)'s handout photo shows containers with a newly registered vaccine against coronavirus in Moscow, Russia on 11 August. Photo:cnn |
Russian business conglomerate Sistema has said it expects to put the vaccine, developed by Moscow’s Gamaleya Institute, into mass production by the end of the year.
According to Russian government officials, the vaccine will be first administered to medical personnel, and then to teachers, on a voluntary basis at the end of this month or in early September. Mass roll-out in Russia is expected to start in October.
The vaccine is administered in two doses and consists of two serotypes of a human adenovirus, each carrying an S-antigen of the new coronavirus, which enter human cells and produce an immune response. The platform used for the vaccine was developed by Russian scientists over two decades and had formed the basis for several vaccines in the past, including those against Ebola.
Is the vaccine effective?
Without data and completed Phase 3 trials, Russia has not proven to the world Sputnik V works.
"I would think that it at least produces antibodies. What we don't know is if it protects people against infection," Neal told CNN.
Russia has said that its vaccine is an adenoviral vector one. Adenoviruses cause the common cold, but in Covid-19 vaccines they're weakened and modified to deliver genetic material that codes for a protein from the novel coronavirus.
The body then produces that protein and may produce an immune response against it but the method can cause problems.
Sputnik V's makers say the vaccine induced a "strong antibody and cellular immune response," in trial volunteers, according to the official website for the vaccine.
"Not a single participant of the current clinical trials got infected with Covid-19 after being administered with the vaccine," the statement adds.
"It's not clear how efficacious the Russian vaccine is going to be and whether or not people have some prior immunity to the adenovirus that they're using to deliver the coronavirus gene sequence," Gottlieb told CNN Tuesday.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, expressed similar concerns.
"I hope that the Russians have actually, definitively proven that the vaccine is safe and effective. I seriously doubt that they've done that," Fauci told Deborah Roberts of ABC News for a National Geographic event to broadcast Thursday. A portion of the interview was posted by National Geographic on Tuesday.
As it stands, the Russian vaccine is unlikely to be meet approval regulations for European Union countries and in the US, will need the FDA's approval.
"The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have fast-track approval procedures for emergency humanitarian use and we need to see evidence that Russia is adopting an equally prudent approach," said Duncan Matthews, Professor of IP Law at Queen Mary University of London, in a statement to the Science Media Center.
"The point is not to be first with a vaccine. The point is to have a vaccine that is safe and effective for the American people and the people of the world," US Health and Human Services Secretary Alex Azar said on ABC Tuesday.
 | Covid-19 no hindrance for a female doctor's weddings in Britain and Malaysia The Covid-19 fear and closure of international borders will not hinder Dr Nur Amalina Che Bakri from planning her double wedding receptions in Britain and ... |
 | Russia unveils COVID-19 vaccine before completing trials Russia has become the first to approve a COVID-19 vaccine, developed by the Gamaleya Institute in Moscow, with production and tens of thousands of inoculations ... |
 | When Covid-19 comes to an end? Although scientists are racing to find a cure for the virus, there's a chance COVID-19 will never fully go away — with or without a ... |
Recommended
 World
World
Thailand Positions Itself As a Global Wellness Destination
 World
World
Indonesia Accelerates Procedures to Join OECD
 World
World
South Korea elects Lee Jae-myung president
 World
World
22nd Shangri-La Dialogue: Japan, Philippines boost defence cooperation
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World