Indian Institute to Assess Made-in-Vietnam Nanocovax Covid-19 Vaccine
 |
| At the signing ceremony in India. Source: Vietnamese embassy in New Delhi |
As part of on-going cooperation between Vietnam and India in the fight against Covid-19, the Translational Health Science and Technology Institute (THSTI), a state-of-the-art centre under the Department of Biotechnology of Government of India, signed a Memorandum of Understanding (MoU) for collaborative research with Nanogen Pharmaceutical Biotechnology (NPB) JSC of Vietnam on Sep. 23 to extend assistance in assessing the immunogenicity of the NanoCovax vaccine which is one of the vaccines being developed in Vietnam against the deadly virus.
The results from THSTI’s assessment would provide critical inputs for NPB to seek emergency use authorization for the NanoCovax vaccine from the Government of Vietnam.
Under the agreement, Nanogen will send cell samples of volunteers participating in the third trial phase of Nanocovax to the THSTI for assessment.
At the event, Secretary of Department of Biotechnology, Government of India, Dr. Renu Swarup sent a congratulatory message. Ambassador of Vietnam in India, Pham Sanh Chau also virtually addressed the event.
Chau affirmed that the collaboration with the THSTI holds significant meaning for Nanogen in the research and development of the vaccine candidate.
The Indian institute’s objective evaluation will help confirm the quality of Nanocovax and serve as a foundation for Nanogen to produce the homegrown Covid vaccine for domestic use as well as for export.
 |
| Indian Ambassador to Vietnam Pranay Verma speaks at the virtual ceremony. Source: Indian embassy in Vietnam |
In his remarks at the virtual ceremony, Indian Ambassador to Vietnam Pranay Verma described the collaboration as a concrete manifestation of the close engagement between and to support each other’s efforts in the fight against Covid pandemic.
30 days after the final sample of Nanocovax was sent, THSTI, one of the 10 Institutes of the Coalition of Epidemic Preparedness for Innovation (CEPI) with the capacity to evaluate the quality of Covid-19 vaccines worldwide, will report the results of Nanocovax's immunogenicity. scientifically and objectively.
The THSTI (of the Department of Biotechnology at the Ministry of Science and Technology of India) is the leading research institute in India with state-of-the-art research facilities for vaccine development, assessment and trials, and important research works in the field of medicine.
Nanogen is working with relevant Vietnamese agencies to step up trials of the vaccine candidate so that it will soon receive approval for emergency use in Vietnam.
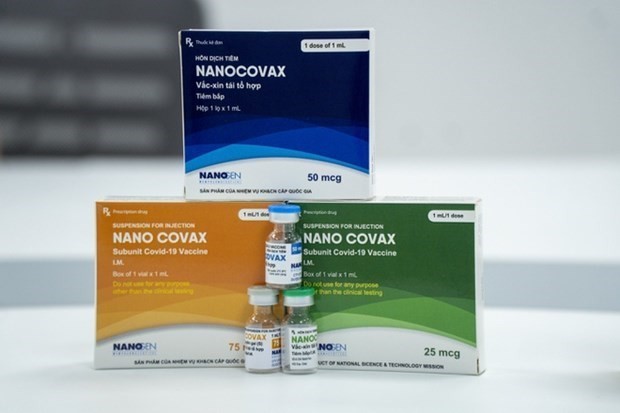 |
| Vietnam's domestically developed coronavirus vaccine NanoCovax. Source: Nanogen |
According to the National Ethics Committee in Biomedical Research's press statement on its conclusions of the mid-term phase 3 trial’s results of Nano Covax, the domestically developed vaccine is deemed to be safe in the short term based on reports of phase 3 trials (during the monitoring of the volunteers’ health seven days after administration of the first dose for 11,430 volunteers, seven days after the administration of second doses for 5,785 volunteers).
The statement, issued on Sep. 19 and reported by VNA, said Nano Covax is deemed to be able to elicit immune responses, based on ongoing phase 3 results – anti-SARS-CoV-2 IgG test results on 924 samples 42 days after first jab; results of neutralising antibodies tests on 761 samples 42 days after first jab; plaque-reduction neutralisation test 42 days after the first jab on 107 samples with the first detected strain of coronavirus, 41 samples with Delta variant, 39 samples with Alpha variant.
The council’s statement stressed that to date they haven’t had the data to “directly" evaluate the protective efficacy, or the ability of a vaccine to prevent infection or symptomatic infections (based on of the number of people infected with Covid after getting the shots), and said more study is needed for assessment of this "most important criteria to determine the vaccine's quality."
The currently available estimation of Nano Covax’s protective efficacy extrapolated from its immunogenicity, or the level of antibodies in the vaccinated, however, has “enough scientific grounds” for the council to send the vaccine documents to the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients for reviews.
Regarding the proposal to grant Nano Covax conditional approval for emergency use, the ethics committee said they reached ‘consensus’ in the use of mid-term phase 3 trials for the drugs advisory body to deliberate.
But it asked the vaccine candidate’s developers to supplement their reports as per the conclusions of the meeting and continue with the trials of the vaccine based on the approved outline to complete trials in March 2022, and give timely updates to the relevant committees and health authorities.
 | Vietnamese Diplomats Help Broken Car in Ladakh, India Vietnamese diplomats in India have helped a broken car during their working trip to the Union Territory of Ladakh. |
 | Celebrity Top Best Dressed at 2021 Emmy With Vietnamese Designer's Jumpsuit Actress Catherine O’Hara top best dressed at the 2021 Emmy Awards with Vietnamese designer's gown. |
 | Vietnam Moves Down 2 Spots to 44th Rank in Global Innovation Index 2021 Vietnam has been ranked 44th by the World Intellectual Property Organization in the Global Innovation Index 2021. |
Recommended
 Friendship
Friendship
Another Vietnamese University Partners with Germany’s Konrad Adenauer Stiftung
 Friendship
Friendship
Over 200 Vietnamese and Russian Children Join “Red Scarf Of Friendship”
 Friendship
Friendship
Venezuela Seeks Vietnam’s Expertise in Science and Technology
 Friendship
Friendship
Diverse Activities to Celebrate the 50th Anniversary of Vietnam - Germany Diplomatic Relations
Popular article
 Friendship
Friendship
Dr. Vu Hoai Chuong Receives Hungary's Knight Cross Order
 Friendship
Friendship
Promoting Vietnam - Japan Economic Cooperation
 Friendship
Friendship
VUFO Attends Fourth Dialogue on Exchange and Mutual Learning among Civilizations
 Friendship
Friendship







