Key things about Russia's second COVID-19 vaccine EpiVacCorona
Russia on Wednesday announced that it has granted regulatory approval to another Covid-19 vaccine named EpiVacCorona. This comes after the country granted similar approval to Sputnik V, making it the first Covid-19 vaccine candidate in the world to attain regulatory approval, India Today said.
"I have a nice piece of news. The Novosibirsk-based Vektor Centre has registered a second coronavirus vaccine, EpiVacCorona," Russian President Vladimir Putin said.
 |
| Russian President Vladimir Putin announces on the second Covid-19 vaccine (Photo: India Today) |
Developed by Siberia-based Vector Institute, EpiVacCorona completed its early-stage human trials in September. The pharmaceutical company is yet to publish the results of human trials and Phase III trial of this vaccine is yet to begin.
"We need to increase production of the first and second vaccine. We are continuing to cooperate with our foreign partners and will promote our vaccine abroad," Putin said.
EpiVacCorona is a peptide-based vaccine and only the second to be licensed for use in Russia. There has been a placebo-controlled trial on 100 volunteers between 18 and 60 in Novosibirsk.
"Novosibirsk-based Vektor Centre has registered a second coronavirus vaccine, EpiVacCorona. Unlike with the first Russian vaccine, Sputnik V, which is an adenovirus vector-based vaccine, the new one is a promising synthetic vaccine based on peptide," the Russian government said.
A shot developed by Moscow's Gamaleya Institute, Sputnik V, was licensed for domestic use in August.
Sputnik V is based on an adenovirus vector and was also registered before Phase III trials. A trial involving 40,000 participants is now underway in Moscow for it.
It is expected that a large-scale human trial of the new vaccine candidate, EpiVacCorona will begin in November or December, TASS news agency reported said citing the consumer safety watchdog Rospotrebnadzor.
This crucial trial is expected to involve nearly 30,000 volunteers, of whom the first 5,000 will be residents of Siberia.
The government said that the peptide-based vaccine does not induce any reactogenic responses and is noted for its high level of safety.
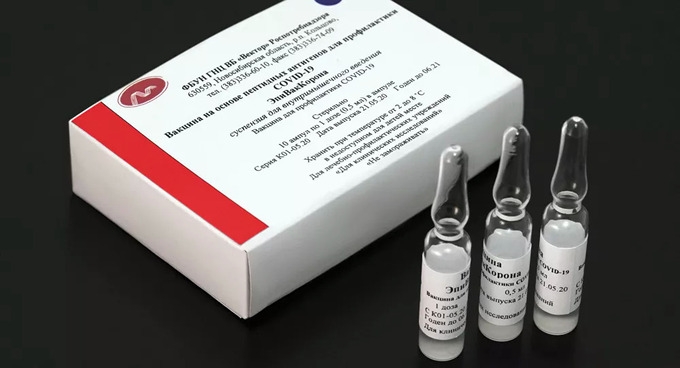 |
| EpiVacCorona vaccine (Photo: Sputnik) |
"The first batch of 60,000 vaccine doses will be produced in the near future, and the Vektor Centre will launch post-registration clinical trials in a number of Russian regions involving 40,000 volunteers. I would like to add that simultaneously the center also plans to start clinical trials on 150 people aged over 60," Deputy Prime Minister Tatyana Golikova was quoted by Zee News. Meanwhile, President Putin also said that there is yet another vaccine that is currently being developed by the Chumakov Institute in St Petersburg. He said it too shall be ready for use soon.
Early-stage clinical trials for this vaccine involving around 300 people will start on October 19.
Key Things to Know About Russia's Second Anti-Coronavirus Vaccine 'EpiVacCorona'
| - The ‘EpiVacCorona’ is a peptide synthetic vaccine based on a recombinant virus. - A peptide synthetic vaccine mimics certain parts of the pathogen they are designed to protect from, compelling the human body to produce antigens capable of fighting the original virus. - According to the Vector Centre, the ‘EpiVacCorona’ vaccine stimulates the intracellular synthesis of coronavirus’s parts. - It forces a body to generate an immune response, both on a cellular level and by releasing antibodies into a person's blood and lymph. - The Vector Centre also said the ‘EpiVacCorona’ vaccine treatment is different from that of ‘Sputnik V’, in terms of the "target group" for the vaccine and in terms of how many injections are required to form the lasting immune response, according to Lastest LT. |
 | Vietnam expected to put COVID-19 vaccine on human trial in 2021 Speaking at the workshop introducing COVID-19 vaccine in Vietnam on September 30, Acting Minister of Health Nguyen Thanh Long said that by 2021, at least ... |
 | High global Covid-19 mortality rate prevents China from completely reopening borders China is unlikely to reopen borders completely as the global mortality rate from Covid-19 remains high. |
 | Vietnam to order COVID-19 vaccines from the US, UK and Russia The Ministry of Health has ordered three COVID-19 vaccine candidates from Russia, the UK, the US. The country’s vaccine research and production speed has also ... |
Recommended
 World
World
India strikes back at terrorists with Operation Sindoor
 World
World
India sending Holy Relics of Lord Buddha to Vietnam a special gesture, has generated tremendous spiritual faith: Kiren Rijiju
 World
World
Why the India-US Sonobuoy Co-Production Agreement Matters
 World
World
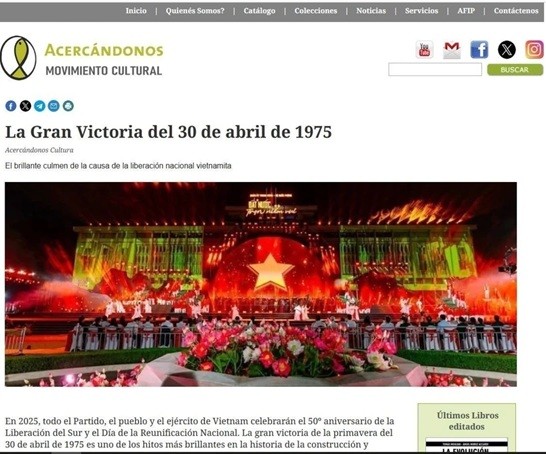
Vietnam’s 50-year Reunification Celebration Garners Argentine Press’s Attention
 World
World
"Will continue offering our full support to Indian govt": US FBI Director after Pahalgam attack
 World
World
"Great Leader": JD Vance Lauds PM Modi During His India Visit
 World
World
Trump’s Tariff Pause: A Strategic Move from “The Art of the Deal”?
 World
World