Massive Coronavirus drugs production face challenges due to high cost of manufacturing
 |
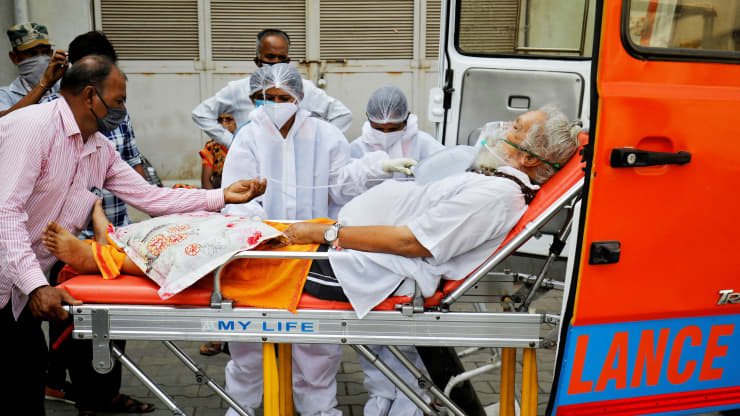
| Demand for medicines that prove their mettle against COVID-19 could swiftly outstrip supply, necessitating new industry alliances, parallel manufacturing by multiple companies and shared intellectual property. | CDC / VIA REUTERS |
“Any pharmaceutical company manufacturing any treatment currently in clinical trials against coronavirus needs a clear plan to upscale production massively,” said Andrew Hill, a research fellow at the University of Liverpool.
“Otherwise, supplies of these drugs could quickly run out.”
Cost of manufacturing medicines for COVID-19 trials
In a study released Friday in the Journal of Virus Eradication, Hill and five other researchers, including Howard University chemist Joseph Fortunak, examined the cost of manufacturing medicines used in recent or ongoing COVID-19 trials.
Using prices for active pharmaceutical ingredients to build estimates, they said Gilead Science’s experimental drug remdesivir, originally made to target Ebola, could be produced for as little as $0.93 (about ¥100) for a day’s supply.
Gilead said the figure did not “accurately reflect” manufacturing costs at scale, but did not give those costs.
Fujifilm Holdings Corp.’s flu drug Avigan runs to $1.45 per day, the researchers said. Fujifilm did not immediately comment.
Meanwhile, decades-old malaria medicine hydroxychloroquine — touted by U.S. President Donald Trump and others as a possible game changer despite no scientific proof it works — costs 8 cents.
Other drugs the researchers examined included the related malaria medicine, chloroquine, the antibiotic azithromycin, Roche’s lung drug Esbriet and rheumatoid arthritis treatment Actemra, as well as an AbbVie HIV drug and a Hepatitis C cocktail.
“Should repurposed drugs demonstrate efficacy against COVID-19, they could be manufactured profitably at very low costs,” the authors wrote, giving range of between $1 and $29 per course of treatment.
Nonetheless, demand for medicines that prove their mettle could swiftly outstrip supply, necessitating new industry alliances, parallel manufacturing by multiple companies and shared intellectual property, Hill said.
Pledges of funding from governments to upscale drug production quantities
Roche, which received $25 million in U.S. funding for Actemra’s COVID-19 trial, said it was ramping up output capacity for intravenous Actemra and had boosted supplies by 50 percent in recent weeks. The Swiss drugmaker added that given Actemra was not yet approved for COVID-19, pricing discussions were premature.
For Fujifilm’s Avigan, Japan provided some $128 million to boost supplies to treat 2 million people, as triple the dose is required for COVID-19 than for influenza.
Gilead can produce 140,000 remdesivir treatment courses near-term, and 1 million-plus by December, it has projected.
Sanofi can make millions of hydroxychloroquine doses, but whether that’s enough may depend on whether trials show it should be used for potentially millions of mildly affected patients, or only for severe ICU patients, Chief Executive Paul Hudson said last week.
Sanofi has boosted production of hydroxychloroquine by 50 percent across its eight manufacturing sites worldwide, and said Friday it would donate 100 million doses to 50 countries should trials show the medicine works, while Novartis has pledged 130 million doses and said it was hunting for more ingredients.
So far, some doctors, including in China, say results have been inconclusive.
Reasons for hope
As of Apr 14, almost 2 million people were infected globally and nearly 120,000 killed by COVID-19, the disease caused by the virus.
While a safe, effective vaccine is still more than a year away, researchers are rushing to repurpose existing drugs and non-drug therapies as well as testing promising experimental drugs that were already in clinical trials, Channel News Asia reported.
Even moderately effective therapies or combinations could dramatically reduce the crushing demand on hospitals and intensive care units, changing the nature of the risk the new pathogen represents to populations and healthcare systems.
New drugs, together with new diagnostics, antibody tests, patient- and contact-tracing technologies, disease surveillance and other early-warning tools, mean the anticipated next wave of the global pandemic does not have to be nearly as bad the first.
More than 70 vaccine candidates are also in development around the world, with at least five in preliminary testing in people.
There are some drugs are on development including Remdesivir - Gilead Sciences, Hydroxychloroquine/chloroquine, Actemra (tocilizumab) - Roche, Kevzara (sarilumab) - Sanofi, Regeneron Pharmaceuticals, Jakavi (ruxolitinib) - Novartis, Incyte, Kaletra (lopinavir/ritonavir) - Abbvie.
 | Latest treatments and drugs for Coronavieus (COVID-19) Latest Treatments and drugs for Coronavieus (COVID-19): Take a look at several of the latest treatments that doctors hope will help fight COVID-19, the disease caused ... |
 | Russia claims to have successfully manufacture three COVID-19 drugs The three drugs are Triazavirin, Favipiravir and the Japanese based Fortepren drug, Vladimir Checkhonin, Vice President of the Russian Academy of Sciences told Sputnik in ... |
 | COVID-19 Florida man claims drug touted by Trump brought him back to life Rio Giardinieri, 52, who was positive for COVID-19 and sent home by hospital, claims he was saved from certain death after taking hydroxychloroquine - an anti-malarial drug ... |
Recommended
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World
India strikes back at terrorists with Operation Sindoor
 World
World
India sending Holy Relics of Lord Buddha to Vietnam a special gesture, has generated tremendous spiritual faith: Kiren Rijiju
 World
World