Thailand prepares to make it own medicine for Covid-19 treatment
| Thai Ambassador: ‘We consider ourselves as Vietnamese’ | |
| Vietnamese in Thailand, RoK donate USD 30,000 to homeland's COVID-19 fight | |
| HUFO presents 10,000 face masks to Thai people |
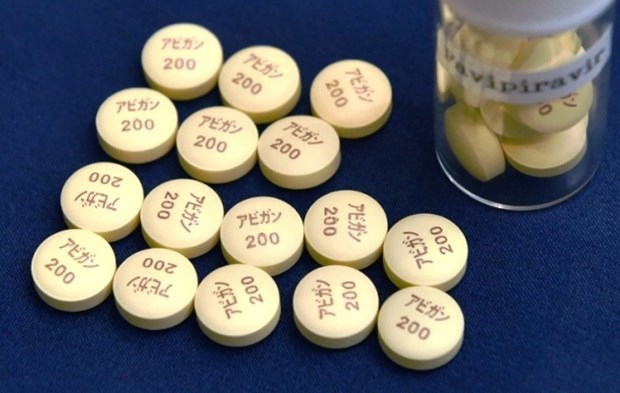 |
| Favipiravir for COVID-19 treatment (Photo: Bangkok Post) |
The Bangkok Post cited Chairman of the GPO's board of directors Sophon Mekthon as saying that the organization’s pharmaceutical team is working hard to study the formula for Favipiravir to make it more efficient, and GPO plans to produce the product by next year.
Thailand has been importing Favipiravir, mostly from Japan, to treat Covid-19 patients with moderate to severe symptoms since January.
The Ministry of Public Health has 200,000 Favipiravir tablets in stock, which is 80% below its target of one million.
But the ministry believes the current stockpile is sufficient to treat 3,000 Covid-19 cases. Due to side effects and limited stock, the ministry limits the use of Favipiravir to more serious infections.
It is not the first time that GPO produced medicine to solve severe outbreaks.
Over a decade ago, the agency produced Oseltamivir under the commercial name GPO-A-Flu® to help combat the bird flu epidemic.
To produce Favipiravir, the agency will seek approval from the Thai Food and Drug Administration (FAD) and the Department of Medical Sciences.
The GPO has already ordered the chemical precursors to produce the drug.
Pitchaya Dilokpattanamongkol, a lecturer at the Faculty of Pharmacy, Mahidol University, said that Favipiravir is not officially recognised as the main drug to fight against Covid-19.
But she touted its "outstanding efficacy" in helping treat the coronavirus while "producing less side effects".
"Many studies found that the drug has produced [limited] side-effects, which can occur in all medicines. There might be an increased risk of uric acid concentrations in the body ... but it is in an acceptable level," Ms Pitchaya said.
But she warned that the Favipiravir is unsafe for pregnant woman because it directly affects the fetus in utero. Ms Pitchaya said that the best way to control Covid-19 would be a vaccine.
There are reportedly 70 medical research teams working to develop such a vaccine. But it is currently believed that an additional 12-18 months will be required before such a vaccine is available, due to the complications stemming from the need for human trials.
According to Nikkei Asian Reviews, Favipiravir, the generic name for Avigan is approved for manufacture and sale in Japan as an influenza antiviral. It selectively inhibits the RNA polymerase of the influenza virus, an enzyme required for viral replication once human host cells are infected. COVID-19 also uses this enzyme to replicate and is classified into the same type of single-stranded RNA virus as influenza; hence, it is believed that Avigan may be effective in treating COVID-19.
Earlier this month, Japan media reports that the country is considering to distribute the influenza antiviral Avigan for free. The medicine developed by Japan's Fujifilm was affirmed "clearly effective in treating coronavirus” by China.
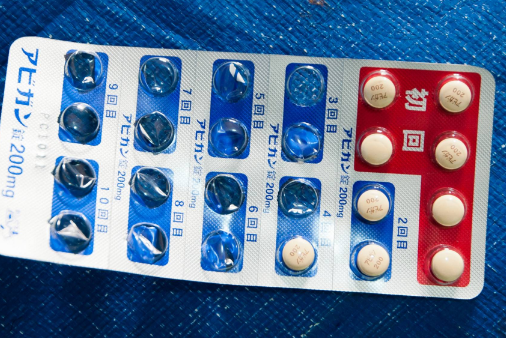 | Experts urge caution when using Avigan as COVID-19 treatment The anti-influenza drug Avigan was promoted as a possible game-changer in the fight against the coronavirus, but medical experts have called for caution, citing its ... |
 | Massive Coronavirus drugs production face challenges due to high cost of manufacturing As countries across the world are hungry for Coronavirus (COVID-19) drugs to beat the pandemic widespread, experts concerns that the production in huge quantities may struggle ... |
 | Japan considers increasing stockpile of Avigan as the drug is tested to treat coronavirus Japan will boost the reserve of Fujifilm Holding Corp’s Avigan anti-flu drug during this fiscal year aiming to serve as many as 2 million people, ... |
Recommended
 World
World
India reports 9 Pakistani Aircraft Destroyed In Operation Sindoor Strikes
 World
World
Thailand Positions Itself As a Global Wellness Destination
 World
World
Indonesia Accelerates Procedures to Join OECD
 World
World
South Korea elects Lee Jae-myung president
 World
World
22nd Shangri-La Dialogue: Japan, Philippines boost defence cooperation
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World





