Experts urge caution when using Avigan as COVID-19 treatment
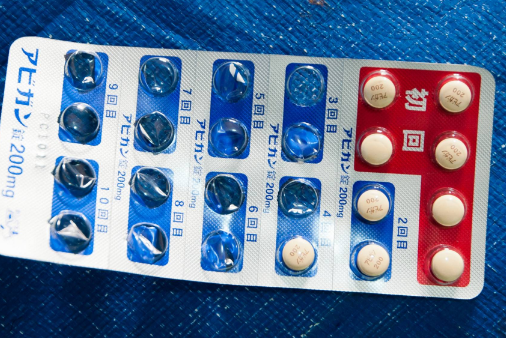 |
| The Avigan anti-influenza drug | DOCTORS WITHOUT BORDERS / VIA KYODO |
A scientific study in China concluded in March that the drug, developed by Fujifilm Toyama Chemical Co., has been effective for patients, especially those with mild symptoms. Beijing has said it will officially adopt the drug as part of its treatment guidelines for COVID-19 patients.
But medical experts warn the drug, also known as favipiravir, may cause birth defects. It cannot be administered to expecting mothers or those who may become pregnant, while careful consideration must be given to allowing men to take it as it is distributed into semen.
As Avigan can inhibit the replication of the virus in cells, experts say it may bring about improvements for those with mild symptoms or those who have recently been infected. It may, however, be much more ineffective for those in whom the virus has already multiplied widely and are experiencing severe symptoms.
They also said Avigan is just one of the options among other candidates as a treatment for the new coronavirus. Those include anti-viral drug remdesivir, developed as a possible treatment for Ebola virus disease, Ciclesonide, a drug used to treat asthma, and Nafamostat, used to treat acute pancreatitis.
Regarding US firm Gilead Sciences Inc.’s remdesivir, The New England Journal of Medicine published a preliminary study by an international team on April 10 showing the drug’s effectiveness in some 70 percent of coronavirus patients with severe symptoms.
“It is not like Avigan has shown exceptionally high effectiveness against the new coronavirus among the candidate drugs,” said Daisuke Tamura, associate professor at Jichi Medical University.
By giving the impression the drug can be used by anyone, the Japanese government may bring about confusion, Tamura said.
“It cannot be helped, to some extent, as we are in a state of emergency but a decision to use the drug should be made carefully by giving a thorough explanation regarding the side effects,” he said.
The Japanese government has also offered to supply the Avigan drug for free to other countries which have shown interest in using it to treat coronavirus patients. Prime Minister Shinzo Abe said over 30 countries have already requested that Japan provide the drug.
At the behest of the government, Fujifilm has begun increasing Avigan production, aiming to secure by September supply to treat up to 300,000 patients per month, approximately a sevenfold increase from March.
Fujifilm started clinical tests of the drug on coronavirus patients in Japan from late March, with results expected around June. Testing is also underway in the United States and Israel.
Due to the possibility that it causes birth defects, the use of Avigan has been strictly managed. It was approved for manufacturing and sales in Japan in 2014.
The drug is considered for use only if the Japan's government approves it as a countermeasure against novel or re-emerging influenza virus outbreaks in which other anti-viral drugs have proven ineffective.
The drug is therefore only manufactured and distributed upon request by the Japan's government and is not distributed in the domestic market or approved for distribution in the United States or in any other country.
Avigan is distinguishable from other anti-influenza drugs as it selectively inhibits the viral RNA polymerase that is necessary for the influenza virus to replicate.
Fujifilm says that due to this mechanism, Avigan may have an anti-viral effect on the new coronavirus because, like influenza, coronaviruses are single-strand RNA viruses that also depend on viral RNA polymerase./.
 | Foreign representative agencies support COVID-19 fight in Japan, Laos To assist the Lao Government’s efforts to fight the outbreak of the coronavirus disease (COVID-19), the Vietnamese Embassy in Laos has raised VND160 million (USD 6,830) ... |
 | COVID-19 fight: 100,000 face masks presented to India The Vietnam-Indian Friendship Association (VIFA) transferred 100,000 face masks to Indian Ambassador to Vietnam Pranay Verma on April 21 to support their fight against the COVID-19 ... |
 | Help vulnerable children get back to school after COVID-19 with The Virtual Steps Challenge The Virtual Steps Challenge is a virtual challenge designed for various participants, with an aim helps disadvantaged children in Vietnam overcome the struggles caused by ... |
 | Can you get reinfected with coronavirus? Recovered coronavirus patients are testing positive again. Can you get reinfected? |
In topics
Recommended
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
Popular article
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World