Vietnam to conduct COVID-19 vaccine trial on willing volunteers
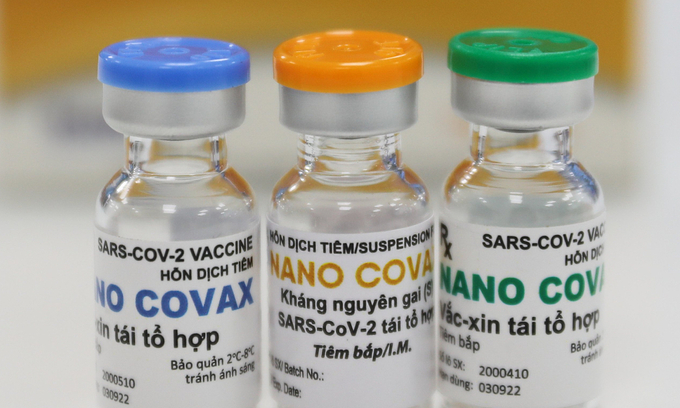 |
| Bottles containing Covid-19 vaccine produced by Nanogen. Photo: VnExpress/Quynh Tran. |
Among the COVID-19 vaccines being studied in Vietnam, the Nanocovac vaccine by Ho Chi Minh City-based Nanogen Pharmaceutical Biotechnology JSC has begun conducting the phase 1 clinical trial, Baotintuc reported.
Regarding the recruitment of volunteers for the vaccine trial, Deputy Head of the Ministry of Health’s Department of Technology Science and Training said “Phase 1 of the COVID-19 vaccine human trial is to seek willing volunteers. These are people who will be given information about the research according to their wanting, and with no pressure regarding health. Volunteers must understand profoundly the trial before injection. Besides, they need to sign a document adopted by the professional council”.
Most importantly, volunteers must ensure medical criteria such as not contracting acute or chronic illnesses as well as having normal indicators of hematology and biochemistry.
All citizens, regardless of ethnicity or geographical conditions are welcomed to partake in the trial, except sensitive ones like pregnant women.
Quang added that this is the experimental stage to evaluate the safety of the vaccine, not yet to assess the immunogenicity. When entering phases 2 and 3, it is needed to recruit people already infectious with the virus.
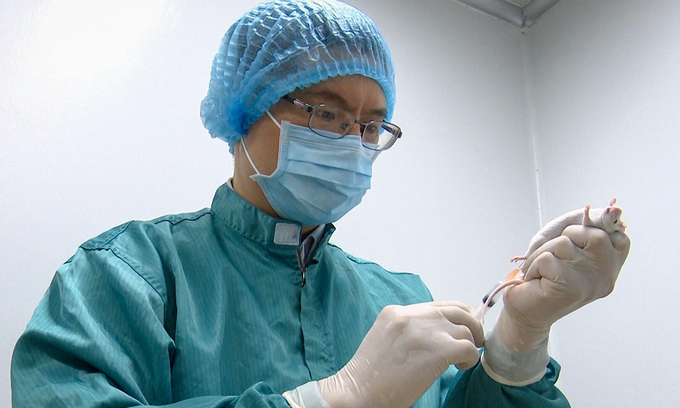 |
| A researcher takes a blood sample from a mouse to test for Covid-19 antibody response. Photo courtesy of Vabiotech. |
According to the Ministry of Health, the domestic COVID-19 vaccine manufacturers are accelerating their work in order to soon carry out the pre-clinical testing. IVAC, VABIOTECH, and NANOGEN completed the production process in the laboratory and is currently assessing the safety and immunogenicity of vaccines on animals. In particular, NANOGEN is ready to carry out the phase 1 clinical trial.
Minister of Health Nguyen Thanh Long requested that one week after recruiting adequate volunteers for the phase 1 trial, the units will inject the first dose of vaccine. He also said that it is necessary for manufacturers and relevant agencies to have readiness for phase 2 of clinical trials.
The Ministry of Health also pledged to create favorable conditions for vaccine researchers such as minimizing administrative procedures and facilitating product registration and licensing.
The Ministry of Health will work with competent agencies to help producers get access to capital for vaccine development and production, he added.
| About 200 vaccine manufacturers around the world have been involved in the development of the COVID-19 vaccine, of which the UK, Germany, and the US have all promised to prepare their vaccines for the domestic market. |
| Vietnam’s current Covid-19 tally stands at 1,381, including four imported cases confirmed on Wednesday night, raising total active cases to 118. The country has gone nine days without recording a new community transmission. |
 | Pfizer-BioNTech’s COVID-19 vaccine meets prescribed success criteria in the clinical study, paving the way for the agency to green-light distribution as early as this weekend. ... |
 | In video: View into Vietnam's COVID-19 vaccine manufacturing factory Nanogen's Nanocovax, a potential COVID-19 vaccine candidate in Vietnam, is entering human trials this week. With qualified researchers, state-of-the-art equipment, Nanogen's vaccine is under prompt ... |
 | Covid-19 vaccination: Many countries begin vaccinations as massive rollout starts Many countries over the world started to roll out coronavirus vaccines to the public. |
Recommended
 Viet's Home
Viet's Home
Kangaroo Mother Care: Simple Approach, Remarkable Results
 Viet's Home
Viet's Home
1,000 Women in Traditional Ao Dai Form Vietnam Map at Hoan Kiem Lake
 Vietnamese Herbal Tea
Vietnamese Herbal Tea
"Enduring Passion" Award Spreads Spirit of Perseverance and Dedication to Vietnam Youth
 Viet's Home
Viet's Home
Embassy Supports Vietnamese people in Northwest Cambodia
 Viet's Home
Viet's Home
Vietnam Marks Significant Milestones in Gender Equality
 Viet's Home
Viet's Home
Good Neighbors Vietnam Assists Students to Pursue their Aspiration
 Viet's Home
Viet's Home
Enhancing Digital Skills of Vietnam's Disadvantaged Youth
 Viet's Home
Viet's Home





