Lastest COVID-19 vaccine updates: FDA says Pfizer-BioNTech safe, effective, China’s Sinovac vaccine is 97 percent effective
 |
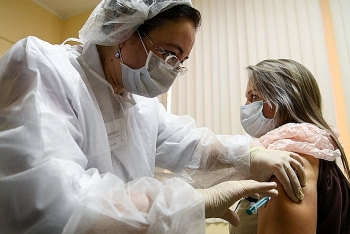
| FDA declares Pfizer's COVID-19 vaccine meets the prescribed success criteria (Photo: Financial Times) |
FDA confirms Pfizer/ BioNTech COVID-19 vaccine safe and effective
The Food and Drug Administration said the first COVID-19 vaccine being considered for U.S. distribution “meets the prescribed success criteria” in a clinical study, paving the way for the agency to green-light distribution as early as this weekend.
In its report Tuesday, the FDA noted that the two-dose vaccine provided benefits even after just the first injection—cutting the risk of getting Covid-19 by about half. The vaccine was found to be 95% effective after the second dose, three weeks later.
According to WSJ, FDA scientists also found that the vaccine was effective in reducing the risk of confirmed severe disease after the first dose, an important finding as some health experts were concerned Covid-19 vaccines would protect against only mild to moderate disease.
The vaccine was effective across ages, weights, and races, although most of the sick subjects were white and many were 55 years old and younger.
FDA's detailed documents about the trials
The proposed dosing regimen for the vaccine is to administer two 30-microgram doses 21 days apart, according to CNN.
However, the document also notes that the vaccine, called BNT162b2, appears to provide "some protection" against Covid-19 following just one dose.
The document describes the efficacy of Pfizer's vaccine in the time between the first and second dose as 52.4%, but the document notes that "the efficacy observed after Dose 1 and before Dose 2, from a post-hoc analysis, cannot support a conclusion on the efficacy of a single dose of the vaccine, because the time of observation is limited by the fact that most of the participants received a second dose after three weeks."
In other words, "the trial did not have a single-dose arm to make an adequate comparison."
 |
| The efficiency rate of Pfizer's vaccine by age group (Source: Pfizer/ via WSJ) |
The document goes on to detail the safety profile of the vaccine as "favorable" and notes that the most common adverse reactions to the vaccine have been reactions at the injection site, fatigue, headache, muscle pain, chills, joint pain, and fever.
Severe adverse reactions occurred in less than 4.6% of participants, were more frequent after the second dose, and were generally less frequent in older adults as compared to younger participants, according to the document. The document adds that swollen lymph nodes also may be related to vaccination.
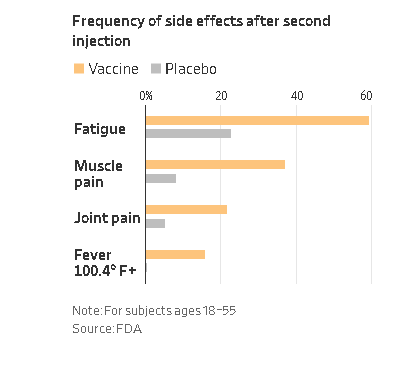 |
| Frequency of side effects after second injection (Source: FDA/ via WSJ) |
A total of six participants died during the trials, and "all deaths represent events that occur in the general population of the age groups where they occurred, at a similar rate."
“It doesn’t mean the vaccine is making you sick, but you should be prepared for potentially not feeling great for a day or two after getting it,” she Dr. Angela Rasmussen, a virologist and affiliate at the Center for Global Health Science and Security at Georgetown University said.
Pfizer/ BioNTech COVID-19 vaccine's promising future
FDA’s announcement is likely to build confidence among health authorities and physicians preparing for a mass vaccination campaign. States, hospitals and other vaccination sites that will receive shots early on are readying themselves and building vaccination teams as Pfizer nears potential distribution.
Pfizer and BioNTech previously said the vaccine was shown to be 95% effective at protecting against symptomatic Covid-19.
With a 95% efficacy, Pfizer’s vaccine exceeded the FDA’s request that a vaccine had to lower the rate of disease by 50% or more when compared with a placebo. The FDA also said that half of the patients in any vaccine study would need to be followed for at least two months after inoculation to ensure that major side effects didn’t occur and that the vaccine’s effectiveness lasted.
If authorized, Pfizer would begin shipping the first of 25 million doses this year, the equivalent for 12.5 million people because it requires two doses. Vaccinations in the U.K. began Tuesday.
Federal officials have estimated that U.S. vaccine deliveries during December will be enough for about 20 million people. That compares with 24 million people in the U.S., such as health-care workers and residents of long-term care facilities, who are in priority groups for vaccines.
A second vaccine from Moderna Inc. is set for a similar review next week.
Chinese Sinovac Covid-19 vaccine is 97% effective
The coronavirus vaccine developed by Chinese company Sinovac is up to 97% effective, according to interim data from tests by Indonesia’s state-owned pharmaceutical company Bio Farma.
“Our clinical trial team found, within one month, that the interim data shows up to 97% for its efficacy,” Bio Farma spokesperson Iwan Setiawan said at a news conference, reports Reuters. Some 1,600 people participated in the clinical trials.
However, the exact efficacy of the vaccine will not be determined until January, according to a Sinovac spokesperson, as these are interim data, as the company is still gathering data on efficacy from the ongoing Phase 3 trial.
According to him, the 97% refers to the seroconversion ratio, which is not necessarily the same as efficiency, as a high seroconversion rate does not necessarily mean that the vaccine effectively protects people against Covid-19.
Unlike Western vaccine manufacturers, the Chinese have so far not released efficiency percentages for the final stage tests, Brussel Times reported.
 | |
|
Globally, there are currently 11 COVID-19 vaccine candidates under the third phase of human trials. Pfizer/BioNTech’s vaccine (the US) is the first vaccine to complete the trials with 95 percent effectiveness and granted the emergency use authorization from the UK and Bahrain.
Meanwhile, Moderna’s vaccine is on its final clinical trial phase, with effective rate reaches 94.5 percent. Oxford/ AstraZeneca is 70-90 percent effective, depending on the injection dose. Russia’s Sputnik V (95 percent effective) is scheduled to begin mass vaccination next week.
Moderna’s vaccine is priced at 37 USD per dose, meanwhile, Pfizer’s vaccine and Oxford’s vaccine are more reasonably priced at 19 USD and 3 UDD per dose, respectively.
 | Vietnam to start human trials of indigenous COVID-19 vaccine in December Nanogen’s COVID-19 vaccine candidate will enter the first stage of human trials starting December 12, Health Minister Nguyen Thanh Long said in a meeting last ... |
 | Made-in-Vietnam Covid-19 vaccine to begin trial on 20 people from Dec 10 After a long time waiting, Vietnam’s Ministry of Health announced that the local Covid-19 vaccine would be trialed from December 10. |
 | UK is world’s first country to approve Pfizer’s COVID-19 vaccine The U.K. has become the first country to approve the use of the Pfizer and BioNTech Covid-19 vaccine, and will begin inoculations next week, Health ... |
Recommended
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World