India refutes reports it declined emergency approval for AstraZeneca's Covid-19 vaccine
“The media report about the rejection of Serum Institute and Bharat Biotech's emergency use authorization of vaccine is fake," news agency ANI quoted Health Ministry as saying.
Broadcasters NDTV and CNBC-TV18 previously reported that India’s Central Drugs Standard Control Organization (CDSCO) had sought more data from the drugmakers after deliberating on applications made this week.
The requests for emergency use of vaccines being manufactured by the Serum Institute and Bharat Biotech were not cleared by a committee of health experts. Both companies have been asked to submit more data at the next meeting, the date of which has not yet been decided.
The final call on the suitability of the vaccine will be taken by the Drug Controller of India or DCGI.
 |
| In India, the Serum Institute of India is manufacturing Covishield and it has already made a request for its emergency use. Photo: AFP |
The Subject Expert Committee of the Central Drugs Standard Control Organisation (CDSCO) met today to review the applications of Pfizer, Serum Institute of India and Bharat Biotech. Pfizer was on the agenda but was not considered today as their experts from the US were not available.
Serum Institute has been asked to submit updated safety data of clinical trials in the country, immunogenicity data from the clinical trial in UK and India and the outcome of the assessment of the UK regulator. Bharat Biotech has been asked for safety and efficacy data from the ongoing Phase III clinical trials in the country.
Approvals are a long process and today's review is only the start, sources said.
Reuters quoted a "source with direct knowledge of the matter" as saying that a decision on the vaccines would be taken "in toto" and it was too early to say whether they would be rejected or accepted.
"It is standard practice for the government to hold several meetings. The process is expected to go on for one or two weeks," sources in Serum Institute said on the development.
| The Pune-based Serum Institute, the world's largest vaccine-maker, requested approval for the Oxford-AstraZeneca vaccine candidate, Covishield, on December 6. Pharma giant Pfizer sought Indian approval after securing clearances in the UK and Bahrain. On Monday, Hyderabad-based Bharat Biotech became the third vaccine-maker to apply to the DCGI for emergency use authorization for its indigenously-developed COVID-19 vaccineCovaxin. Prime Minister Narendra Modi visited the vaccine facilities in Hyderabad and Pune last month to review the progress of the Covid shots. At an all-party meeting on December 4, PM Modi expressed hope that a COVID-19 vaccine may be ready in a few weeks. |
Oxford vaccine Covishield shows 70% efficacy against Covid-19
Peer-reviewed analysis of the Oxford vaccine confirms that the second group which received a low first dose of vaccine demonstrated 90% efficacy, while the first group with standard dose showed 62.1% efficacy. Overall efficacy, at least 14 days after the second dose of the vaccine was therefore calculated to be 70.4%.
Researchers at the University of Oxford who have been involved in the development of the varsity's Covid-19 vaccine candidate - Covishield - on Tuesday published the first peer-reviewed results of a Phase 3 trial of the vaccine candidate, the Newindianexpress reported.
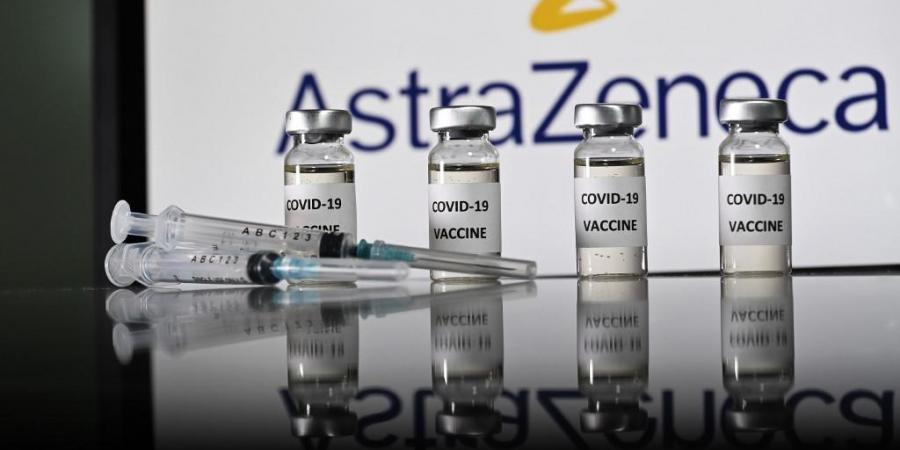 |
| An illustration picture shows vials with Covid-19 Vaccine stickers attached and syringes with the logo of British pharmaceutical company AstraZeneca. Photo: AFP |
During peer review, research work is analyzed by neutral experts who are not associated with the vaccine development of its trials. Peer-reviewed analysis of Phase 3 trials of the Oxford vaccine tested across two different dose regimens has been published by leading medical journal Lancet.
"The efficacy data are based on 11,636 volunteers across the United Kingdom and Brazil and combined across three groups of vaccinated people vaccinated," Oxford researchers said in a statement.
Among the two groups, one received a standard dose followed by a booster dose, while the second group received a low first dose vaccination followed by a standard second dose.
The peer-reviewed analysis of the trial confirms that the second group which received a low first dose of vaccine demonstrated 90.0 percent efficacy, while the first group with standard dose showed 62.1 percent efficacy.
The peer-reviewed analysis of the trial confirms that the second group which received a low first dose of vaccine demonstrated 90.0 percent efficacy, while the first group with standard dose showed 62.1 percent efficacy.
Professor Andrew Pollard, Director of the Oxford Vaccine Group and Chief Investigator of the Oxford Vaccine Trial, said, "Today, we have published the interim analysis of the Phase 3 trials and show that this new vaccine has a good safety record and efficacy against the coronavirus. We are hugely grateful to our trial volunteers for working with us over the past eight months to bring us to this milestone."
 |
| Vials of AstraZeneca's Covishield Photo: Hindustantimes |
The trial's safety database notes that only three cases of trials-related fever were found. Out of these, two people had received the vaccine.
According to the authors, "One of them was directly considered possibly related to the vaccine."
No person who received the Oxford vaccine had a severe disease or needed hospitalization.
Of the 11,636 volunteers, only five cases occurred in those who were more than 55 years old during the primary analysis, Newindianexpress added.
"The vaccine efficacy in older age groups will be determined in future analyses after more cases have accrued in this age range," the researchers said.
Professor Sarah Gilbert, Professor of Vaccinology at the University of Oxford who led the team of scientists on the vaccine, said, "We have known for many years that adenoviral vectored vaccines fulfill the requirements for use against outbreak or pandemic diseases. They are safe, highly immunogenic, can be manufactured in large quantities at low cost, and do not require frozen storage. Following the demonstration of vaccine efficacy in many preclinical studies, we now have clear evidence of efficacy in the trial results presented in a peer-reviewed publication today. Now under regulatory review, we hope that this vaccine will shortly be in use to start saving lives."
The researchers are hopeful that this data might also suggest that the low dose/standard dose vaccine may provide protection against asymptomatic infection, but stressed that these data are at an early phase.
Pascal Soriot, Chief Executive Officer of AstraZeneca, the drug manufacturer with whom Oxford University has collaborated for developing the vaccine, said, "Today's peer-reviewed publication enables a full disclosure of the Oxford program interim analysis. The results show that the vaccine is effective against Covid-19, with in particular no severe infections and no hospitalizations in the vaccine group, as well as safe and well-tolerated. We have begun submitting data to regulatory authorities around the world for early approval and our global supply chains are up and running, ready to quickly begin delivering hundreds of millions of doses on a global scale at no profit."
Six out of 249 volunteers drop out ahead of the second dose in Mumbai
Six volunteers who were participating in the clinical trial of the Oxford-AstraZeneca vaccine for Covid-19, Covishield, at the King Edward Memorial (KEM) hospital in Parel, Mumbai, have dropped out. However, at the BYL Nair Hospital, which is another center, all volunteers have participated in the trial, Hindustantimes reported.
At KEM Hospital, a total of 101 people had participated in the trial which began on September 26 in Mumbai. The hospitals started administering the dosage in the latter half of October. But when called for the second dose that was to be administered 28 days later, only 95 returned. “It is a voluntary process, we cannot force them if they don’t want to come,” said Dr. Hemant Deshmukh, hospital dean.
 |
| An employee in personal protective equipment (PPE) removes vials of AstraZeneca's COVISHIELD, coronavirus disease (COVID-19) vaccine from a visual inspection machine inside a lab at Serum Institute of India, Pune, India, November 30, 2020. Photo: Reuters. |
Recently, a 40-year-old man from Chennai who had participated in the trial complained of developing cognitive issues after taking the vaccine. However, the hospital had no information on why these six volunteers dropped out. Dr. Deshmukh said, “We don’t know why they dropped out of the trial. It is a personal choice.”
At Nair hospital, 148 volunteers have participated in the clinical trial of the same vaccine. “At our hospital, all volunteers have completed both the dosages,” said Dean Dr. Ramesh Bharmal.
Both the hospitals have completed administering the two doses last week, and are now monitoring the immune responses and health of the participants. So far, no one has reported any adverse effects. The trial duration is 180 days.
| As reported by Forbes, the vaccine candidate CovidShield is a licensed version of the Oxford-AstraZeneca vaccine, and the firm plans to produce 1 billion doses of five separate vaccine candidates by the end of their 2021-22 financial year. Earlier this year, the Serum Institute of India (SII) — the world’s largest vaccine producer — inked a deal with AstraZeneca which allows the Indian firm to make a billion doses of the Oxford vaccine for India and lower- and middle-income countries during the pandemic and sell it at the cost of production. The firm has a similar license manufacturing deal for Novovax’s vaccine candidate and is also developing its own vaccine. Bharat Biotech’s Covaxin has been domestically developed by the pharmaceutical firm in partnership with the Indian Council of Medical Research (ICMR). On Tuesday, India’s health ministry had said that some Covid-19 vaccines are likely to receive emergency authorization in the next few weeks. |
 | Covid-19 vaccination: Many countries begin vaccinations as massive rollout starts Many countries over the world started to roll out coronavirus vaccines to the public. |
 | Vietnam’s COVID-19 vaccine to be priced under US $22 Nanogen’s COVID-19 vaccine Nanocovax is expected to be priced at no more than VND 500,000 (US $21.62) each dose and can be covered by health ... |
 | Lastest COVID-19 vaccine updates, global experts are optimistic about vaccine development While provincial governments across China are placing orders for experimental, domestically made coronavirus vaccines, UK to administer the first dose of Pfizer's vaccine this week, ... |
Recommended
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World





