Vietnam’s COVID-19 vaccine to be priced under US $22
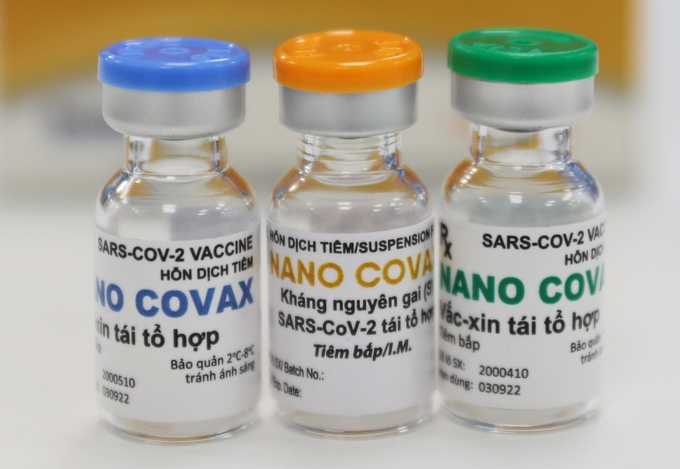 |
| Nanocovax is a potential COVID-19 vaccine candidate in Vietnam (Photo: VNE) |
“Though we have yet to reach agreement with the Ministry of Health, we’re sure that each dose won’t exceed US $21,62”, a representative from Nanogen company was quoted by VNE as saying Tuesday morning.
The representative further stated that Nanocovax was reasonably priced and affordable to all Vietnamese. The company is trying to include the vaccine into the list of drugs covered by health insurance.
With Nanocovax, each person aged 12-75 will get two injections, the second one is 28 days following the first one. Immunization is expected to last for about one year, citizens must get repeated injections.
Nanogen’s COVID-19 vaccine is evaluated as safe. Side effects on mice and monkeys are “negligible”, only cause mild irritation and itching which last for only 30 minutes. Anatomy of vaccinated mice found no internal organ damages.
The health ministry earlier has assessed Nanogen’s Covid-19 vaccine candidate among the most promising, having been successfully produced it on a laboratory scale and provoked immunogenicity during animal testing.
“Nanogen is capable of rolling out 2 million doses per year. In the coming 6 months, we’ll upgrade the capacity by an additional 30 million doses, ideally 50 million doses a year”, the company said.
 |
| Vietnamese researchers at Vabiotech company working on COVID-19 vaccine (Photo: VNE) |
 |
| COVID-19 vaccine developers at Vabiotech company (Photo: VNE) |
Nanocovax is the first made-in-Vietnam COVID-19 vaccine to enter human trials. Nanogen partners with the Vietnam Military Medical Academy to start recruiting volunteers participating in the first phase of the human trial starting December 10. The volunteers will be given the first test shots of the vaccine a week later, as reported by VNE.
According to experts, those who are selected for participation in the COVID-19 vaccine trials must be healthy volunteers who are suffering from no underlying illnesses and have no medical history. These individuals will be carefully questioned about their health history, along with any allergies to drugs, food, or other things before they get vaccinated, as reported by VOV.
The second phase, which involves 400 volunteers, is expected to start three months after the first phase.
Nanocovax is scheduled to go into mass vaccination in May 2021.
 |
| A Vietnamese researcher working on COVID-19 vaccine (Photo: VNA) |
| Vietnam has four Covid-19 vaccines produced by Nanogen, Vabiotech, Polyvac and the Institute of Vaccines and Medical Biologicals (IVAC) currently under research. Vabiotech, Polyvac are currently evaluating their vaccines on animals, having completed the laboratory-scale production process. Meanwhile, IVAC will continue to cooperate with Russia and “actively contact with China to have access to China’s vaccine”. |
| On global scale, there are currently 11 COVID-19 vaccine candidates under the third phase of human trials. Pfizer/BioNTech’s vaccine (the US) is the first vaccine to complete the trials with 95 percent effectiveness and granted the emergency use authorization from the UK and Bahrain. Meanwhile, Moderna’s vaccine is on its final clinical trial phase, with effective rate reaches 94.5 percent. Oxford/ AstraZeneca is 70-90 percent effective, depending on the injection dose. Russia’s Sputnik V (95 percent effective) is scheduled to begin mass vaccination next week. Moderna’s vaccine is priced at 37 USD per dose, meanwhhile Pfizer’s vaccine and Oxford’s vaccine are more resonably priced at 19 USD and 3 UDD per dose, respectively. |
 | What is mRNA-1273 - Moderna COVID-19 vaccine? The drugmaker Moderna announced highly encouraging results on Monday, saying that complete data from a large study show its mRNA-1273 vaccine to be 94.1 percent ... |
 | Gold price hits 4-month low as vaccine progress slams precious metals Gold fell to its lowest in four-months on November 25 as December gold futures last traded at US$1,809.50 an ounce on the day. |
 | COVID-19 vaccines could get green light from EU regulator late 2020, early 2021 Europe's medicines regulator said Monday that it could give the green light to the first coronavirus vaccines late this year or early next. |
Recommended
 National
National
Vietnam News Today (May 12): Party General Secretary Meets With Russian Experts, Intellectuals
 National
National
Vietnam News Today (May 11): Vietnam, Austria to Boost Cooperation in High-Tech Development, Innovation
 National
National
Vietnam News Today (May 10): Vietnamese Peacekeepers Honored with UN Medal in South Sudan
 National
National
Vietnam News Today (May 9): Vietnam Ready to Work With Russia to Elevate Relations
 National
National
Vietnam News Today (May 8): Vietnam Remains Committed to UNCLOS
 National
National
Vietnam News Today (May 7): Vietnam Hosts Over 7.67 Million International Visitors in First 4 Months
 National
National
Vietnam News Today (May 6): Party Leader To Lam Meets Vietnamese Expatriates in Kazakhstan
 National
National