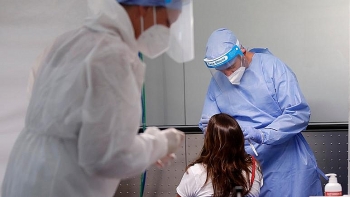
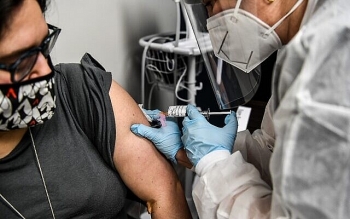
WHO cautious on COVID-19 plasma upon US's authorization on emergency treatment
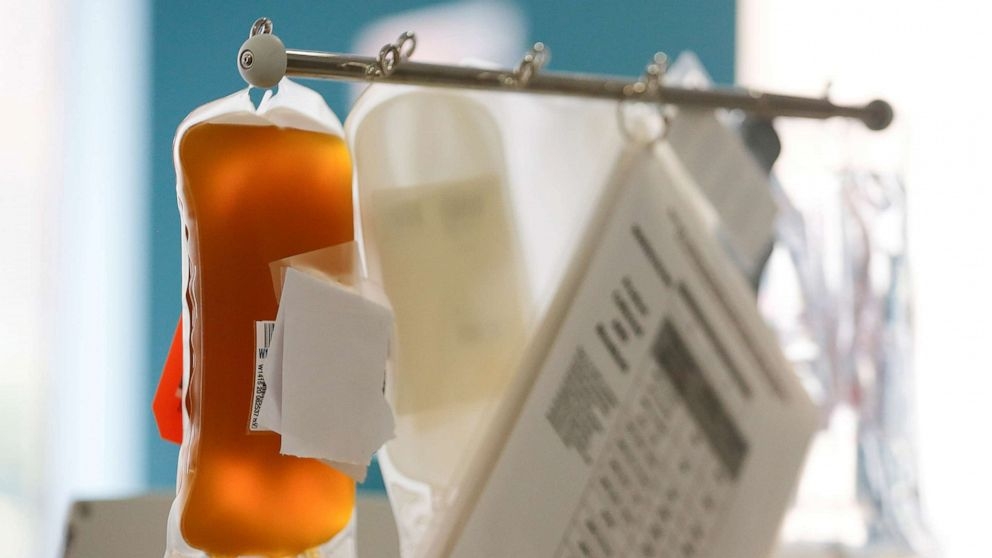 |
| WHO was cautious about endorsing the use of recovered COVID-19 patients' plasma to treat those who are ill (Photo: Reuters) |
So-called convalescent plasma, which has long been used to treat diseases, has emerged as the latest political flashpoint in the race to find therapies for COVID-19, as reported by Reuters.
The U.S. Food & Drug Administration (FDA) on Sunday authorized its use after President Donald Trump blamed the agency for impeding the roll-out of vaccines and therapeutics for political reasons.
The technique involves taking antibody-rich plasma from patients who have recovered from COVID-19 and giving it to those who are suffering from severe active infections in hopes they will recover more quickly.
Soumya Swaminathan, WHO chief scientist, said only a few clinical trials of convalescent plasma have produced results, and the evidence, at least so far, has not been convincing enough to endorse it beyond use as an experimental therapy. While a few trials have showed some benefit, she said, they have been small and their data, so far, inconclusive.
“At the moment, it’s still very low-quality evidence,” Swaminathan told a news conference. “So we recommend that convalescent plasma is still an experimental therapy, it should continue to be evaluated in well-designed randomized clinical trials.”
Evidence is conflicting: One Chinese study showed plasma from people who have recovered from coronavirus failed to make a difference in hospitalized patients, while another, pooled analysis showed it can lower the risk of death.
One challenge, Swaminathan added, was plasma’s variability, since it is drawn from many different people, producing a product that is less-standardized than monoclonal antibodies crafted in the lab.
World Health Organization senior adviser Bruce Aylward added that beyond plasma’s efficacy, there were also potential safety risks that must be vetted.
“There are a number of side effects,” Aylward said, ranging from mild fevers to severe lung injuries or circulatory overload. “For that reason, the clinical trial results are extremely important.”
The U.S. National Institutes of Health this month announced it was giving several million dollars toward a mid-stage convalescent plasma trial.
 |
| WHO General Director Tedros Adhanom Ghebreyesus (Photo: AP) |
| Convalescent plasma is an antibody-rich product made from blood donated by people who have recovered from the disease caused by the virus. Prior experience with respiratory viruses and limited data from other countries suggest that convalescent plasma has the potential to lessen the severity or shorten the length of illness caused by COVID-19. The EUA had earlier authorized the distribution of COVID-19 convalescent plasma in the United States and its administration by health care providers, as appropriate, to treat suspected or laboratory-confirmed COVID-19 in hospitalized patients with COVID-19, said the FDA. The announcement, however, raises concerns among medical experts. Many said that the emergency authorization could make it more difficult to perform the very trials necessary to study the treatment, abc News reported. |
| In Vietnam, experts have started to study plasma therapy for COVID-19 patients since April. The research project related to the use of plasma in COVID-19 treatment was approved by Vietnamese Ministry of Health on August 3. |
 | China authorises emergency usage of select domestic Covid-19 vaccines An emergency use authorization of COVID-19 vaccine, which based on Chinese vaccine management law, allows select unapproved vaccine candidates from domestic companies to be used ... |
 | Chinese Covid-19 vaccine’s price ten times higher than Russian’s According to the head of a major state-owned Chinese pharmaceutical company Sinopharm, its coronavirus vaccine may cost some hundred yuan per shot and around 1,000 ... |
 | Vaccine production: a billion-dollar industry According to estimates of research firm Alliance Bernstein (headquarter in the US), the global vaccine production industry is worth $ 52 billion per year. In ... |
Recommended
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
Popular article
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World
India strikes back at terrorists with Operation Sindoor
 World
World
India sending Holy Relics of Lord Buddha to Vietnam a special gesture, has generated tremendous spiritual faith: Kiren Rijiju
 World
World