Determining Vietnam’s COVID-19 vaccine position on world’s vaccine map?
 |
| Over 50 nations are joining the race to produce Covid-19 vaccine (Photo: Getty Images) |
COVID-19 vaccine race in the world
Vaccine’s effective rate must reach at least 70 percent (according to WHO) and 50 percent (according to FDA) to be qualified for authorization.
Russia is the world’s first nation to submit its vaccine Sputnik V for emergency approval since the pandemic broke out. The country has started vaccination program as soon as August on over 100,000 residents. On November 24, Sputnik V was pronounced with 91.4 percent effective.
In late November, US’s Moderna and UK’s AstraZeneca also announced their Covid-19 vaccine candidate to be 94.1 percent and 70 percent, respectively. Two vaccine manufacturers Johnson & Johnson and Novavax are planning to release their trial data in early 2021.
On December 3, the UK became the first nation in the world to ever roll out its Pfizer-BioNTech vaccine for mass vaccination. The US, Canada, Singapore, Baren, Mexico, France and EU had followed suit. Pfizer-BioNTech vaccine is over 90 percent effective though, its harsh storing and transport requirements (minus 70 degrees Celcius) make a big drawback for the candidate.
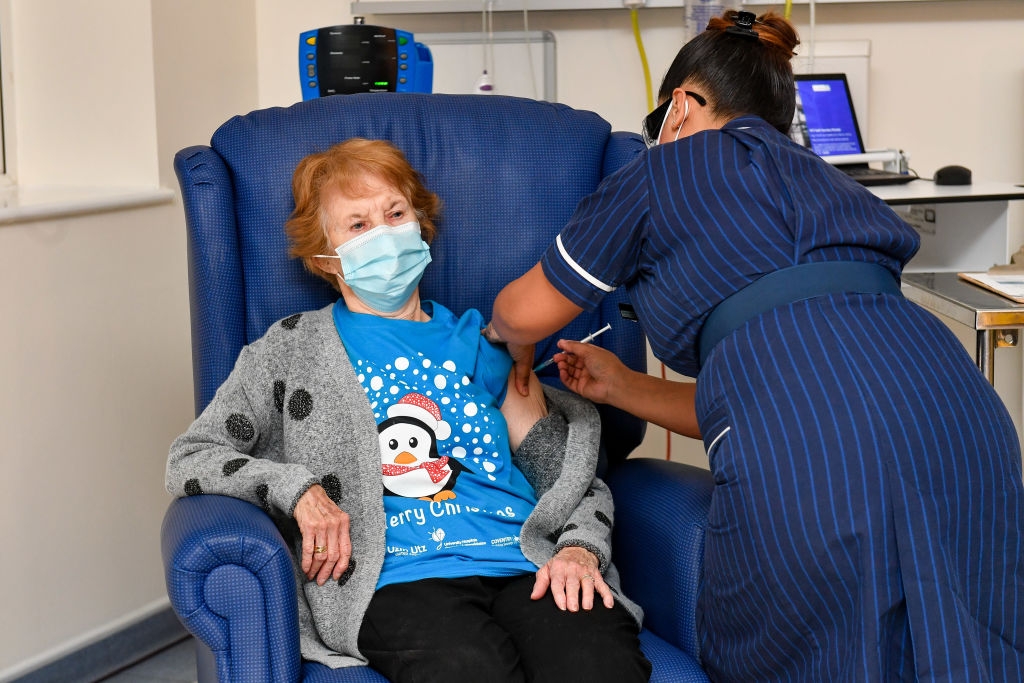 |
| First British citizen to receive the much-awaited Pfizer-BioNTech Covid-19 jab (Photo: Time Magazine) |
China, as a country in the forefront of the COVID-19 vaccine race, also announced to the world its candidate – developed by China National Biotec Group Co. – is 79.34 percent effective against the novel virus. The trial over 31.000 volunteers in UAE also proves the vaccine is over 86 percent effective, no serious complications reported. The vaccine is awaiting approval from China’s National Medical Products Administration.
COVID-19 vaccines in Vietnam
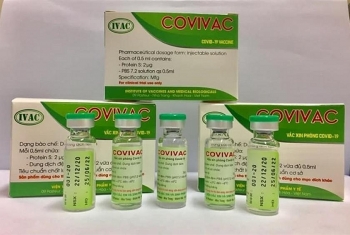
Meanwhile, in Vietnam, the country’s been showered with praises over comprehensive and effective COVID-19 containment attainment is having four potential vaccines on hands. The vaccines are studied and produced by Nanogen, Vabiotech, Polyvac and the Institute of Vaccines and Medical Biologicals (IVMB), all have completed the laboratory production process.
While Vabiotech and Polyvac’s vaccines are still under evaluation on animals, IVMB’s candidate Covivac is scheduled to enter human trials this January after yielding safe and strong immunity response on animals.
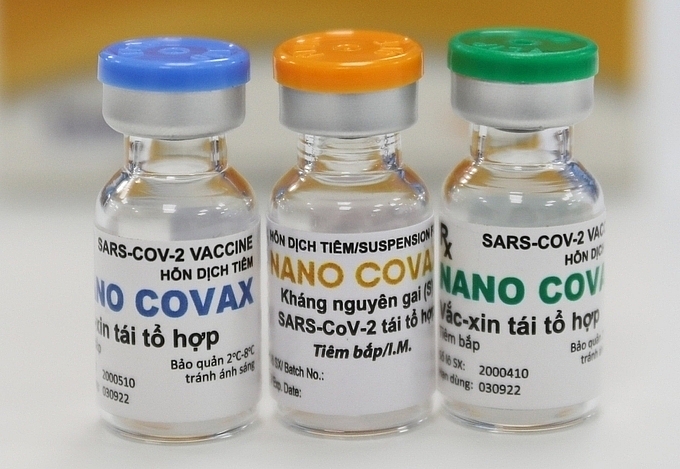 |
| Vietnam's NanoCovax vaccine (Photo: VNE) |
As reported by VOV, IVAC will cooperate with the National Institute of Hygiene and Epidemiology and Hanoi Medical University to trial the vaccine on 125 volunteers aged 18-59. Those receiving IVAC’s trial jabs must be healthy, having no underlying disease and satisfying several other specific criteria.
According to Dr. Duong Huu Thai, Director of IVAC, the Institue has been studying Covivac since last May, aiming at successfully producing the vaccine and completing three human trials within 18 months. The first phase of the trails is scheduled to finish in April.
In the meantime, Nanocogen’s Nonacovax vaccine has gone half the way into the first phase of human trials. Nanogen Biopharmaceutical company is expected to end its Nanocovax vaccine’s human trials by February 2022.
The human clinical trials protocol, which includes three phrases, was approved by the Ethics Council of the Ministry of Health on December 9. Each phrase consists of two injections, 28 days apart.
The first phase aiming at testing the safety of Nanocovax started on December 10 and is scheduled to end in February 2021. It was conducted in Hanoi on 60 volunteers aged 18-50. The first three volunteers who received the jab on December 17 showed no abnormal symptoms to date.
The second phase is planned to start right after the first one and last for 6 months. 400-600 volunteers aged 12-75 will be recruited for the trial, which evaluates the immunogenicity and preventive potency of the vaccine.
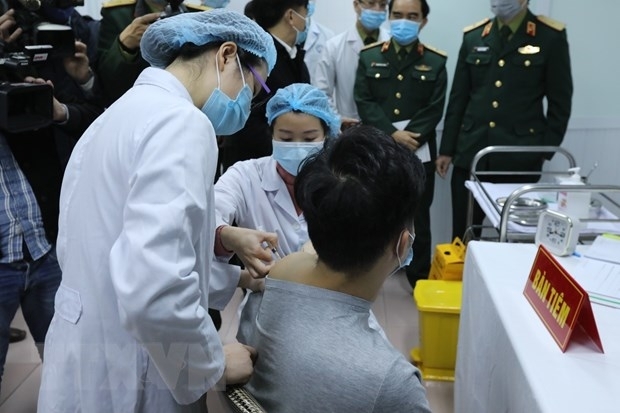 | |
|
The third phase (August 2021 - February 2022) is planned to take place in an epidemiological area in India, Indonesia, or Bangladesh to gather enough 1,500 – 3,000 volunteers aged 12-75.
Researchers said they might kick off the second phase earlier right after the first phase yields 50 percent effective. Thus, according to Do Minh Si, Development Research Director of Nanogen, the clinical trials might end at the end of 2021.
Nanocovax is priced at VND120,000 ($5.17) per dose.
Along with injections, Vietnam’s COVID-19 Nanocovax vaccine will also be developed in the form of eye-drop and nasal spray for special subjects.
At Nanocovax's manufacturing establishment (Video: VNE)
| Deputy Minister of Health Truong Quoc Cuong on January 4 confirmed that Vietnam has signed with BioPharmaceutical Company AstraZeneca for 30 million doses of its Covid-19 vaccine. The shipment would guarantee enough vaccinations for at least 15 million Vietnamese people. With 7 new imported Covid-19 patients, the national count of Covid-19 cases is raised to 1,504, including 693 locally transmitted cases. The number of recovered patients is now 1,339 while fatalities remain at 35. Among patients still under treatment, nine have tested negative for SARS-CoV-2 once, six twice and five thrice. A total of 19,286 people who had close contact with Covid-19 patients or returned from pandemic-hit areas are being quarantined nationwide. |
 | Vietnam in talks with UK to buy 30 million doses of Covid-19 vaccine The Ministry of Health is negotiating with British Covid-19 vaccine developers AstraZeneca and Oxford University to purchase 30 million doses of Covid-19 vaccine. |
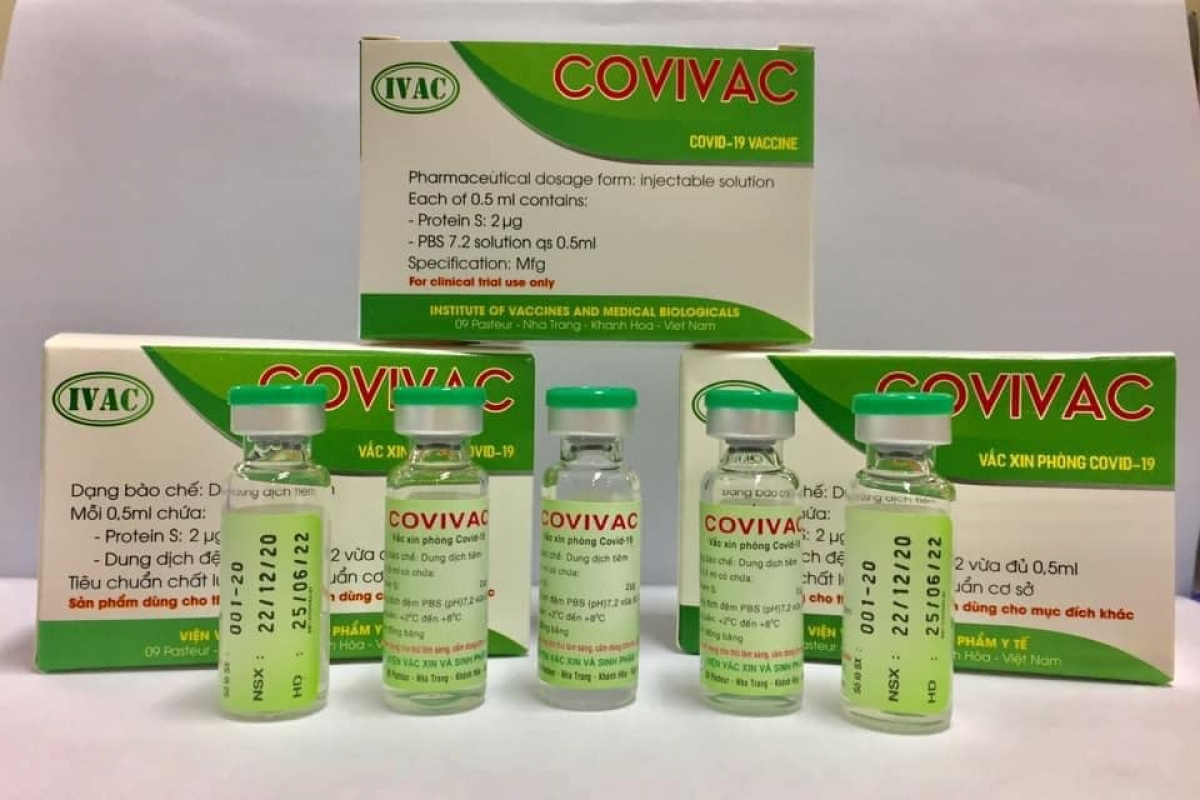 | Vietnam's human trials of Second Covid-19 vaccine may begin in January Human trials of second homegrown Covid-19 vaccine may begin in late January this year. The Ministry of Health has granted permission to the Nha Trang-based ... |
 | India follows UK to grant emergency approval for AstraZeneca/Oxford Covid-19 vaccine India has followed the U.K. and granted emergency approval for the coronavirus vaccine developed by AstraZeneca Plc and the University of Oxford, the first step ... |
Recommended
 National
National
Vietnam News Today (May 9): Vietnam Ready to Work With Russia to Elevate Relations
 National
National
Vietnam News Today (May 8): Vietnam Remains Committed to UNCLOS
 National
National
Vietnam News Today (May 7): Vietnam Hosts Over 7.67 Million International Visitors in First 4 Months
 National
National
Vietnam News Today (May 6): Party Leader To Lam Meets Vietnamese Expatriates in Kazakhstan
 National
National
Boarding Kindergarten Children Supported with VND360.000/Month
 National
National
Vietnam News Today (May 5): Party Chief To Lam’s Trip to Russia Carries Special Strategic Significance
 National
National
Vietnam News Today (May 4): Vietnam, Sri Lanka Deepen Traditional Friendship, Comprehensive Cooperation
 National
National