Post AstraZeneca injection reaction rate in Vietnam “within expected level”
 | All COVID-19 vaccinated people in Vietnam are in stable health condition |
 | Over 5,000 Vietnamese front-liners vaccinated against COVID-19 |
 | Vietnam Airlines offers free COVID-19 vaccine transportation |
 |
A vial containing a Covid-19 vaccine by AstraZeneca is ready for use in Gia Lai Province in Vietnam's Central Highlands. (Photo: VNE) |
“There’s always a certain chance of side effects following injection. The rate in Vietnam is within the expected level as determined by vaccine producers, World Health Organization and other relevant entities”, Dang Duc Anh, director of the National Institute of Hygiene and Epidemiology, said on Monday.
He stated that globally around 26 percent of COVID-19 vaccinated individuals experience normal side effects, 0.7 percent would display severe reactions. In Vietnam, the latter registers 0.1 percent – the rate recorded 8 days into the AstraZeneca campaign which is rolling out in 12 provinces and cities, as reported by VNE.
Among over 16,000 front liners already receiving the first dose, medical authorities confirm 11 recipients developing severe side effects (high fever, increased blood pressure) after the AstraZeneca shot. All had had their overall health checked prior to vaccination, no underlying disease reported and are in stable health condition now.
The Ministry of Health has established specialized committees to investigate the causes of these severe anaphylaxis. The ministry also asked localities to continue to monitor and evaluate the post-injection response status.
According to the World Health Organization’s recommendations (WHO), about 1-10 percent of people vaccinated with COVID-19 vaccine develop “common reaction”, including swelling and redness at the injection site; more than 10 percent have a “very common reaction” including headache, nausea, muscle pain, joint pain, itching, fatigue, restlessness, low-grade fever, chills, pain, and heat at the injection site.
Serious reactions such as grade 3 anaphylaxis, late hypersensitivity reactions are yet to be fully-reported by WHO.
None have experienced blood clots after vaccination, a problem that has prompted at least 10 countries.
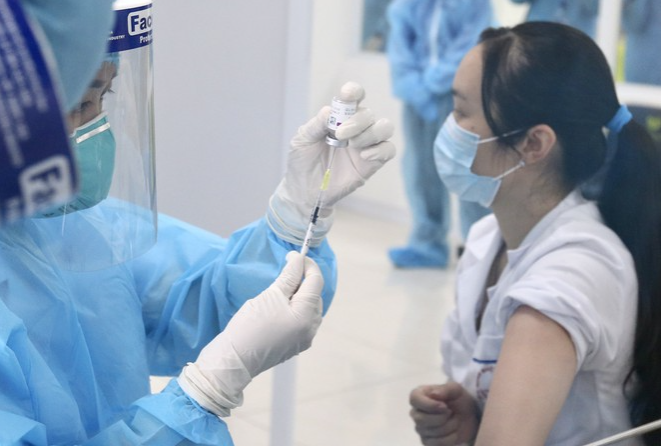 |
| (Photo: Tien Phong) |
 |
| (Photo: Vietnam Plus) |
“Vietnam would continue to implement the vaccination program as planned. Recipients would have their health closely monitored after the injection”, Professor Dang Duc Anh, Director of the Central Institute of Hygiene and Epidemiology (NIHE), Head of the Steering Committee of the Expanded Immunization Project, was quoted by VNE as saying March 12 morning.
The blood clots complication post AstraZeneca injection have reported in several countries in Europe. Following the incident, 9 European countries, including Denmark, Norway, Iceland on March 11, 12 announced to suspend or ban AstraZeneca's vaccine in their COVID-19 inoculation programs. Local authorities are investigating the new dangerous complication.
The European Medicines Agency (EMA), however, advocates the use of the UK’s candidate. It said there are just 30 cases displaying blood clots out of over 5 million vaccinated individuals in Europe. Scientists are currently scrutinizing every report of side-effects post AstraZeneca inoculation.
The UK-developed vaccine has been used in 25 nations across the world, including Vietnam. So far, statistics said the most common reactions after getting the vaccine, accounting for over 10 percent, are headache, nausea, muscle pain, joint pain, tenderness, fatigue, restlessness, fever, chills, etc. Less than 10 percent develop redness and swelling at the injection site. Reports from 4 clinical trials with nearly 24,000 volunteers show these symptoms would disappear within 7 days. Such reactions after the second dose are at a much lower rate than the first one.
AstraZeneca and European Medicines Agency have said there has been no evidence to prove the vaccine caused blood clots, and stressed its benefits far outweigh the risk of contracting Covid-19.
| The Vietnam Vaccine JSC (VNVC) has ordered 30 million Covid-19 vaccine doses from British-Swedish firm AstraZeneca, with the first batch of 117,600 doses having arrived last month. The batch, produced by South Korean firm SK Bioscience, is being kept in VNVC cold storage to be distributed to 13 cities and provinces with recent coronavirus outbreaks. While exact fees have yet to be disclosed, a $30 million deposit as per the firm's purchase contract has been confirmed. VNVC and AstraZeneca first began negotiations back in the second quarter of 2020, when the AstraZeneca vaccine had entered its third phase of human trials. For the past six months, VNVC has been developing its own cold chain and storage equipment to house the vaccines, besides training personnel on how to handle them properly, VNE reported. Its handover means the ministry will actively handle the vaccination process. Besides the 30 million doses ordered, Vietnam would receive an additional 4.1 million, also from AstraZeneca, via global vaccine access mechanism Covax by April. Another 25.9 million doses would be transported to Vietnam, also through Covax, between August and November. Vietnam is also negotiating with other vaccine manufacturers in the U.S., Russia and some other countries to ensure it could obtain a total of 150 million doses this year to cover 70 percent of its population. |
 | Vietnam will take initiative in COVID-19 vaccine availability With two promising homegrown COVID-19 vaccine candidates, Vietnam is expecting vaccine availability in late 2021, early 2022. |
 | In Pictures: Made-in-Vietnam Covivac vaccine's human trial procedures All volunteers get general health checks, have their blood samples and pregnancy test taken before getting the indigenous Covivac vaccine Monday morning at Hanoi Medical ... |
 | China eases entry of foreigners inoculated with its home-grown Covid-19 vaccines Starting from today (March 15), China will facilitate foreigners who have been inoculated with China-produced COVID-19 vaccines applying to enter the Chinese mainland via Hong ... |
Recommended
 National
National
Vietnam News Today (May 31): Vietnam Strongly Supports Laos’s National Development
 National
National
Vietnam News Today (May 30): Vietnam, Venezuela Reinforce Ties Through People-to-people Diplomacy
 National
National
Vietnam News Today (May 29): Vietnam and Hungary to Expand Cooperation into New Areas
 National
National
Vietnam News Today (May 28): Vietnam and China Discuss Strategic Cooperation Orientations
Popular article
 National
National
Vietnam News Today (May 27): Vietnam Treasures Multifaceted Collaboration with France
 National
National
Vietnam Commits to Building an Inclusive, Sustainable and Cohesive ASEAN
 National
National
Vietnam Proposes Vision for Responsible Digital Journalism Cooperation
 National
National