Russia unveils COVID-19 vaccine before completing trials
 |
| Russia has become the first to approve a coronavirus vaccine (Photo: Clinical Trials Arena) |
The development paves the way for the mass inoculation of the Russian population, even as the final stage of clinical trials to test safety and efficacy continue, Reuters reported.
Speaking at a government meeting on state television, Putin said the vaccine, developed by Moscow’s Gamaleya Institute, was safe and that it had even been administered to one of his daughters.
“I know that it works quite effectively, forms strong immunity, and I repeat, it has passed all the needed checks,” said Putin, adding that he hoped the country would soon start mass-producing the vaccine.
According to Washington Post, Kirill Dmitriev, head of the Russian Direct Investment Fund that bankrolled the country’s vaccination effort, said that the vaccine will be named “Sputnik,” a reference to the first orbital satellite, which was launched by the Soviet Union and started the great Cold War space race.
Its approval by the health ministry foreshadows the start of a larger trial involving thousands of participants, commonly known as a Phase III trial.
Such trials, which require a certain rate of participants catching the virus to observe the vaccine’s effect, are normally considered essential precursors for a vaccine to receive regulatory approval.
 |
| President Vladimir Putin (Photo: Infonet) |
Regulators' concerns about Russia's vaccine
Regulators around the world have insisted that the rush to develop COVID-19 vaccines will not compromise safety. But recent surveys show growing public distrust in governments’ efforts to rapidly-produce such a vaccine.
Russian health workers treating COVID-19 patients will be offered the chance of volunteering to be vaccinated soon after the vaccine’s approval, a source told Reuters last month.
More than 100 possible vaccines are being developed around the world to try to stop the COVID-19 pandemic. At least four are in final Phase III human trials, according to WHO data.
According to Washington Post, officials have pledged to vaccinate millions of people, including teachers and front line health-care workers, with the experimental coronavirus vaccine beginning this month, raising global alarm that the country is jumping dangerously ahead of critical, large scale testing that is essential to determine if it is safe and effective.
Russian officials have said that a second vaccine from the state research center in Siberia, Vector, is not far behind.
“Of course, what counts most is for us to be able to ensure the unconditional safety of the use of this vaccine and its efficiency in the future. I hope that this will be accomplished,” Putin said at a meeting with government members Tuesday, adding that his own daughter had been inoculated with the Gamaleya vaccine.
The aggressive strategy from a country eager to declare a victory amid one of the worst outbreaks in the world has been criticized by outside scientists who worry that shots could be harmful or give people a false sense of security about their immunity. China has already authorized one vaccine for use in its military, ahead of definitive data that it is safe and effective.
The leading Russian vaccine candidate has so far been tested in small, early clinical trials designed to find the right dose and assess any safety concerns. It was given to scientists who developed it — in self-experimentation that is unusual in modern science — 50 members of the Russian military and a handful of other volunteers.
Dmitriev said last week Russia will go ahead with Phase III for the Gamaleya vaccine once it is registered by the country’s Health Ministry. In addition to the Russian trial, parallel ones will also be conducted in Saudi Arabia, the United Arab Emirates and possibly Brazil, he said. But while that testing is still ongoing, Russia intends to start vaccinating willing front-line medical workers and teachers, who will be asked to document how they’re feeling.
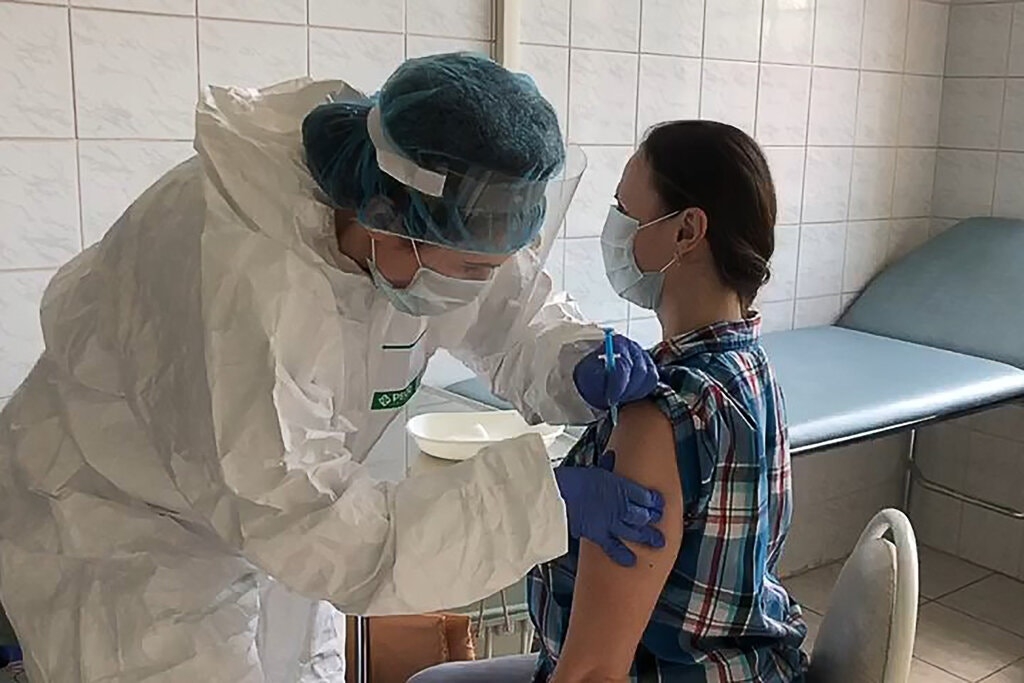 |
| A volunteer receives the vaccine developed by the Gamaleya Institute as part of clinical trials (Photo: Getty Images) |
The World Health Organization still lists the Gamaleya vaccine as being in Phase I. Asked about Russia’s vaccine developments, WHO spokesman Christian Lindmeier told reporters in Geneva last week that any vaccine should go “through all the various trials and tests before being licensed for rollout,” adding that “between finding or having a clue of maybe having a vaccine that works, and having gone through all the stages, is a big difference.”
Russia’s vaccine uses two doses to deliver different harmless cold viruses, or adenoviruses, that have been engineered to carry into cells the gene for the spiky protein that studs the outside of the coronavirus. The approach is also being used by scientists at the pharmaceutical giant Johnson & Johnson, the Chinese company CanSino Biologics and the University of Oxford in their vaccine candidates.
But those other efforts have published data on how vaccines perform in animals that range from mice to monkeys, and also presented data from early human trials showing the severity of any reactions, ranging from soreness at the injection site to fevers. The CanSino vaccine uses one of the same harmless viruses the Russians are using in its vaccine, and its results have been disappointing to some scientists.
 | When Covid-19 comes to an end? Although scientists are racing to find a cure for the virus, there's a chance COVID-19 will never fully go away — with or without a ... |
 | Researchers determine materials making least effective masks for Covid-19 prevention Researchers at Duke University in the US launched an experiment to determine which types of masks are quite literally useless in shielding people from Covid-19 ... |
 | COVID-19 Updates (August 10) : Russia to register world's first coronavirus vaccine The clinical trial data and other documents of Russia's COVID-19 vaccine are currently under expert review. The decision on registration will be made based on ... |
Recommended
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World





