U.S. to Pay $1 Billion for 100 Million Doses of a COVID-19 Vaccine Candidate
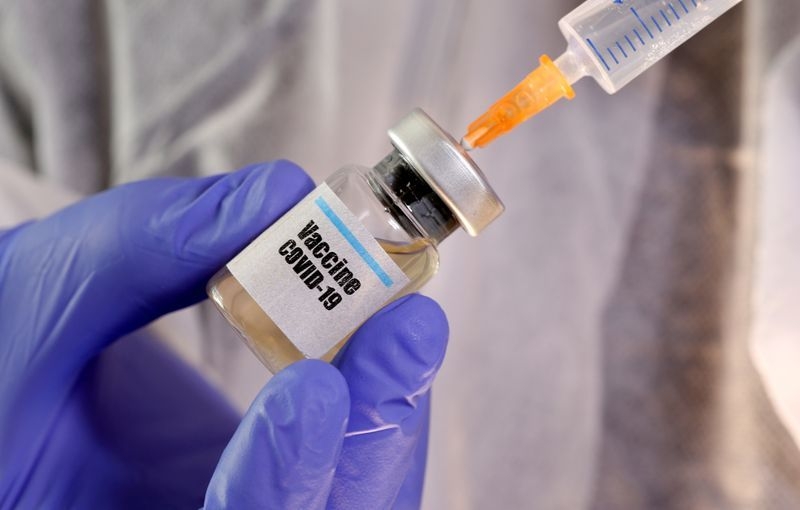 |
| The new vaccine will be soon available for the US market. Photo: Reuters |
Johnson & Johnson on August 05 announced its Janssen Pharmaceutical Companies have entered into an agreement with the U.S. government for the large scale domestic manufacturing and delivery in the U.S. of 100 million doses of Janssen’s SARS-CoV-2 investigational vaccine, Ad26.COV2.S, for use in the United States following approval or Emergency Use Authorization by the U.S. Food and Drug Administration (FDA).
The Biomedical Advanced Research and Development Authority (BARDA), in collaboration with the U.S. Department of Defense, is committing over $1 billion for this agreement. The vaccine will be provided at a global not-for-profit basis for emergency pandemic use. The U.S. government may also purchase an additional 200 million doses of Ad26.COV2.S under a subsequent agreement.
“Johnson & Johnson’s global team of experts has worked tirelessly alongside BARDA and scientific partners to pursue a SARS-CoV-2 vaccine that can help to stop the spread of COVID-19", said Paul Stoffels, Vice Chairman of the Executive Committee and Chief Scientific Officer, Johnson & Johnson.
Johnson & Johnson’s efforts to develop a SARS-CoV-2 vaccine have been undertaken pursuant to an ongoing research and development collaboration with BARDA and under the oversight of the FDA. Based on the positive preclinical data recently published in the peer-reviewed journal Nature, the Phase 1/2a first-in-human clinical trial of the vaccine candidate, Ad26.COV2.S, is underway in healthy volunteers in the United States and Belgium.
 |
| Johnson & Johnson has been working hard with partners to pursue a SARS-CoV-2 vaccine. Photo: News18 |
The Company is evaluating one- and two-dose regimens, in its clinical program and working diligently to ensure broad, global access to the vaccine following approval or authorization by regulators. Johnson & Johnson aims to meet its goal to supply more than one billion doses globally through the course of 2021, provided the vaccine is safe and effective.
Johnson & Johnson’s SARS-CoV-2 vaccine program leverages Janssen’s AdVac® technology. The same technology was used to develop Janssen’s European Commission-approved Ebola vaccine and construct its HIV, RSV, and Zika vaccine candidates. More than 90,000 individuals have been vaccinated to date using the Janssen AdVac®-based platform.
According to Reuters, there are currently no approved vaccines for COVID-19. More than 20 are in clinical trials and Moderna's candidate among the farthest along in development.
| According to Aljazeera, Moderna announced on July 27 that it had started a late-stage trial to test the effectiveness of its vaccine candidate - the first such trial under Operation Warp Speed and that last hurdle before regulatory approval. Moderna's study will test the response to the vaccine in 30,000 adults who do not have the respiratory illness. The company, which has never brought a vaccine to market, has received nearly $1bn from the US government, which has chosen it as one of the first to enter large-scale human trials. The late-stage trial is designed to evaluate the safety of Moderna's mRNA-1273 and to determine if the vaccine can prevent symptomatic COVID-19 after two doses. The study also seeks to answer if the vaccine can prevent death caused by COVID-19, and if just one dose can prevent symptomatic COVID-19. Moderna said it is on track to deliver about 500 million doses a year, and possibly up to one billion doses a year, beginning 2021.
|
 | Bill Gates denies conspiracy theories he created virus outbreak Bill Gates pushed back against some of the conspiracy theories spreading online accusing him of creating the coronavirus outbreak. |
 | COVID-19 vaccine's expected to have in Vietnam soon Vietnam expects to have a safe and effective Covid-19 vaccine in October 2021 when shortening the reviewing, appraising and approving of clinical trial process as well ... |
 | Latest COVID-19 Vaccine: Oxford-AstraZeneca study shows positive immune action A coronavirus vaccine by the University of Oxford and AstraZeneca Plc showed positive results in the early trial on humans, a promising progress in the COVID-19 ... |
Recommended
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World
India strikes back at terrorists with Operation Sindoor
 World
World
India sending Holy Relics of Lord Buddha to Vietnam a special gesture, has generated tremendous spiritual faith: Kiren Rijiju
 World
World
Why the India-US Sonobuoy Co-Production Agreement Matters
 World
World
Vietnam’s 50-year Reunification Celebration Garners Argentine Press’s Attention
 World
World



![More than 150 coronavirus vaccine candidates are in various stages of development, with 23 prospects in human trials across the globe and Moderna's candidate among the farthest along in development [Brian Snyder/Reuters] 1259 3504ea7d89f541ac98b28ccb0b158bf6 18](https://vietnamtimes.org.vn/stores/news_dataimages/vanthuanvnt/082020/05/22/1259_3504ea7d89f541ac98b28ccb0b158bf6_18.jpg?rt=20200806064254)



