Vaccines/drugs in the pipeline for COVID-19 treatment
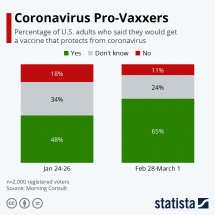
| When coronavirus vaccines available for use? | |
| Coronavirus Vaccine: Global Competition | |
| China claims that Coronavirus vaccine could be ready by April |
 |
| Scientist Xinhua Yan works in the lab at Moderna in Cambridge, Massachusetts, which has developed the first experimental coronavirus medicine, on February 28, 2020. But an approved treatment is more than a year away. David L. Ryan/The Boston Globe via Getty Images |
The mysterious coronavirus outbreak in the Chinese city Wuhan, now termed as COVID-19, and its fast spread to many other countries, endangers thousands of lives. The pandemic has catalysed the development of novel coronavirus vaccines across the biotech industry, both by pharmaceutical companies and research organisations such as the National Institutes of Health (NIH), US.
The first COVID-19 vaccine in China is expected to be ready for clinical trials by the end of April, according to Xu Nanping, China’s vice-minister of science and technology. Inovio Pharmaceuticals plans to begin clinical trials on a coronavirus vaccine in April this year.
Health officials from WHO have noted that Gilead’s remdesivir has demonstrated efficacy in treating the coronavirus infection.
Chloroquine to be tested for coronavirus treatment in the US
The President of the United States, Donald Trump, announced on 19 March that chloroquine (hydroxychloroquine/Plaquenil), a drug used to treat malaria and arthritis, was approved by the US Food and Drug Administration (FDA) to be tested as a treatment for COVID-19. Chloroquine is being tested in various clinical trials conducted by government agencies and academic institutions.
Other antivirals drugs are also planned to be fast-tracked for testing for coronavirus.
Favilavir, the first approved coronavirus drug in China
The National Medical Products Administration of China has approved the use of Favilavir, an anti-viral drug, as a treatment for coronavirus. The drug has reportedly shown efficacy in treating the disease with minimal side effects in a clinical trial involving 70 patients. The clinical trial is being conducted in Shenzhen, Guangdong province.
Pharmaceutical companies involved in developing coronavirus drugs/vaccines
Here is a list of the major coronavirus drugs that pharmaceutical companies across the world are developing that have the potential to become major coronavirus vaccines or antivirals for treating the contagious coronavirus infection.
Novel coronavirus vaccines
Listed below are the coronavirus vaccines in various stages of development, across the world.
TJM2 by I-Mab Biopharma
I-Mab Biopharma is developed TJM2, a neutralising antibody, as a treatment for cytokine storm in patients suffering from a severe case of coronavirus infection. The drug targets the human granulocyte-macrophage colony-stimulating factor (GM-CSF), which is responsible for acute and chronic inflammation.
The company will commence development after receiving approval for the Investigational New Drug (IND) application from the U.S. Food and Drug Administration (FDA).
Coronavirus vaccine by Medicago
Medicago is developing drug candidates against COVID-19 after having produced Virus-Like Particles (VLP) of the coronavirus. The company has formed a collaboration with Laval University’s Infectious Disease Research Centre to develop antibodies against SARS-CoV-2.
The company’s research activities are being partly funded by the Canadian Institutes for Health Research (CIHR).
AT-100 by Airway Therapeutics
Airway Therapeutics is exploring its novel human recombinant protein named AT-100 (rhSP-D) as a treatment for coronavirus. The company has announced a filing with the Respiratory Diseases Branch of the National Institutes of Health to evaluate the drug.
AT-100 has shown efficacy in preclinical studies in reducing inflammation and infection in the lungs, while also generating an immune response against various respiratory diseases.
TZLS-501 by Tiziana Life Sciences
Tiziana Life Sciences is developing its monoclonal antibody named TZLS-501 for the treatment of COVID-19. TZLS-501 is a human anti-interleukin-6 receptor (IL-6R), which helps in preventing lung damage and elevated levels of IL-6.
The drug works by binding to IL-6R and depleting the amount of IL-6 circulating in the body thereby reducing chronic lung inflammation.
OYA1 by OyaGen
OyaGen’s OYA1 has shown strong antiviral efficacy against coronavirus in laboratory essays. It was found to be more effective than chlorpromazine HCl in inhibiting SARS-CoV-2 from replicating in cell culture.
OYA1 was earlier approved as an investigational new drug for treating cancer but abandoned due to lack of efficacy. OyaGen plans to conduct further research on the drug to determine the efficacy in treating coronavirus.
BPI-002 by BeyondSpring
BeyondSpring’s BPI-002 is a small molecule agent indicated for treating various infections including COVID-19. It has the ability to activate CD4+ helper T cells and CD8+ cytotoxic T cells and generating an immune response in the body.
If combined with another COVID-19 vaccine, the drug has the ability to generate long-term protection against viral infections. BeyondSpring has filed US patent protection for the drug for treating viral infections.
Altimmune’s intranasal coronavirus vaccine
An intranasal Covid-19 vaccine is being developed by US-based clinical-stage biopharmaceutical company, Altimmune.
Design and synthesis of the single-dose vaccine have been completed, while animal testing will follow.
The coronavirus vaccine is being developed based on a vaccine technology platform that is similar to NasoVAX, an influenza vaccine developed by Altimmune.
INO-4800 by Inovio Pharmaceuticals and Beijing Advaccine Biotechnology
Inovio Pharmaceuticals has collaborated with Beijing Advaccine Biotechnology Company to advance the development of the former’s vaccine, INO-4800, as a novel coronavirus vaccine. The company has started pre-clinical testing for clinical product manufacturing.
The vaccine development is supported by a $9m grant from the Coalition for Epidemic Preparedness Innovations (CEPI).
Inovio announced an accelerated timeline for the development of the vaccine on 03 March. Preclinical trials are ongoing and the design for human clinical trials have been completed. The company has also prepared 3,000 doses for human clinical trials planned to be conducted across the US, China, and South Korea. Plans for large-scale manufacturing have also been developed.
Human clinical trials in 30 healthy volunteers are expected to commence in April 2020 in the US, followed by China, and South Korea. A phase one clinical trial is planned to be conducted in parallel in China, by Beijing Advaccine. Results from the clinical trials are expected to be available in September 2020.
Inovio aims to produce one million doses of the vaccine by the end of 2020 to perform additional clinical trials or emergency use.
NP-120 (Ifenprodil) by Algernon Pharmaceuticals
Algernon Pharmaceuticals has announced that it is exploring its NP-120 (Ifenprodil) as a potential treatment COVID-19. Ifenprodil is an N-methyl-d-aspartate (NDMA) receptor glutamate receptor antagonist sold under the brand name Cerocal. It has demonstrated efficacy in improving survivability in mice infected with H5N1.
APN01 by University of British Columbia and APEIRON Biologics
A drug candidate developed by APEIRON Biologics named APN01 is being tested in China in a phase one pilot trial as a treatment for COVID-19. APN01 is based on research conducted by a professor at the University of British Columbia for treating SARS. The research revealed that the ACE2 protein was the main receptor for the SARS virus.
The clinical trial will test the drug’s efficacy in reducing the viral load in patients. Data from the trial will be used to determine if additional clinical trials are required to be conducted in larger number of patients.
mRNA-1273 vaccine by Moderna and Vaccine Research Center
Moderna and the Vaccine Research Center, a unit of the National Institute of Allergy and Infectious Diseases (NIAID), have collaborated to develop a vaccine for coronavirus. The vaccine targets the Spike (S) protein of the coronavirus.
The first vials of the vaccine have been manufactured at Moderna’s Massachusetts manufacturing plant and shipped to NIAID for phase one human clinical trial. The trial began on 16 March at the Kaiser Permanente Washington Health Research Institute in Seattle, Washington. A total of 45 males and females aged between 18 and 45 have been enrolled for the trial.
The participants will be divided into three cohorts who will be administered 25 microgram (mcg), 100mcg or 250mcg dose 28 days apart.
Avian Coronavirus Infectious Bronchitis Virus (IBV) vaccine by MIGAL Research Institute
The MIGAL Research Institute in Israel announced that an Infectious Bronchitis Virus (IBV) vaccine developed to treat avian coronavirus has been modified to treat COVID-19. The vaccine has demonstrated efficacy in pre-clinical trials conducted by the Volcani Institute.
The IBV vaccine was developed after four years of research and has high genetic similarity to the human coronavirus. The institute has genetically modified the vaccine to treat COVID-19 and will be available in the oral form.
The institute is currently exploring potential partners for producing the vaccine in the next eight to ten weeks and obtaining the necessary safety approvals for in-vivo testing.
TNX-1800 by Tonix Pharmaceuticals
Tonix Pharmaceuticals has partnered with Southern Research, a non-profit research organisation, to develop a coronavirus vaccine named TNX-1800. The vaccine is a modified horsepox virus developed using Tonix’s proprietary horsepox vaccine platform.
TNX-1800 is designed to express a protein derived from the virus that causes the coronavirus infection. Southern Research will be responsible for evaluating the efficacy of the vaccine, under the partnership.
Brilacidin by Innovation Pharmaceuticals
Innovation Pharmaceuticals announced that it is evaluating Brilacidin, a defensin mimetic drug candidate, as a potential treatment for coronavirus. Brilacidin has shown antibacterial, anti-inflammatory and immunomodulatory properties in several clinical trials.
The company is planning to explore research collaborations and seek federal grants to develop the coronavirus drug. It is already investigating the drug for inflammatory bowel disease and oral mucositis in cancer patients.
Innovation has signed two material transfer agreements with a university in the US and 12 biocontainment labs in the US for evaluation of Brilacidin as a treatment for COVID-19. One of the biocontainment labs is scheduled to commence testing of the drug in the third week of March.
Recombinant subunit vaccine by Clover Biopharmaceuticals
Clover Biopharmaceuticals is developing a recombinant subunit vaccine using its patented Trimer-Tag© technology. The company is developing the vaccine based on the trimeric S protein (S-Trimer) of the COVID-19 coronavirus, which is responsible for binding with the host cell and causing a viral infection.
Using Trimer-Tag© technology, Clover successfully produced the subunit vaccine in a mammalian cell-culture based expression system on 10 February. The company also identified antigen-specific antibody in the serum of fully recovered patients who were previously infected by the virus.
A highly purified form of the S-Trimer vaccine is expected to be available in six to eight weeks for performing pre-clinical studies. The company is equipped with in-house cGMP biomanufacturing capabilities to scale-up production if the vaccine is proven to be successful.
Clover is also collaborating with GSK to develop a vaccine using the latter’s pandemic adjuvant system.
Vaxart’s coronavirus vaccine
Vaxart is developing an oral recombinant vaccine in tablet formulation using its proprietary oral vaccine platform, VAAST.
The company plans to develop vaccines based on the published genome of 2019-nCOV to be tested in pre-clinical models for mucosal and systemic immune responses.
CytoDyn-leronlimab
CytoDyn is examining leronlimab (PRO 140), a CCR5 antagonist, as a potential coronavirus drug.
The drug is already being investigated in phase two clinical trials as a treatment for HIV and has been awarded fast-track approval status by the United States Food and Drug Administration.
Linear DNA Vaccine by Applied DNA Sciences and Takis Biotech
Applied DNA Sciences’ subsidiary LineaRx and Takis Biotech formed a joint venture on 07 February to develop a linear DNA vaccine as a treatment for coronavirus. The JV will use Polymerase Chain Reaction (PCR)-based DNA manufacturing technology to develop the vaccine.
The PCR technology offers several advantages including high purity, increased production speed, and absence of antibiotics and bacterial contaminants. Further, the vaccine gene developed through this technology can be effective without being inserted into the patient’s genome.
The design for four DNA vaccine candidates is expected to be produced using the PCR technology for carrying out animal testing. The design of one of the vaccine candidates is based on the entire spike gene of the coronavirus, while the remaining are designed based on the antigenic portions of the protein.
BXT-25 by BIOXYTRAN to treat late-stage acute respiratory distress syndrome (ARDS)
BIOXYTRAN announced that it is exploring partners to develop its lead drug candidate, BX-25, as a treatment for Acute Respiratory Distress Syndrome (ARDS) in late-stage patients infected with the coronavirus. The diffusion of oxygen to the blood is comprised in patients suffering from ARDS leading to fluid build-up in the lungs.
BX-25 is designed to be 5,000 times smaller than blood cells and efficiently transport oxygen through the body for a period of nine hours before being processed by the liver. The drug can help in supplying oxygen to the vital organs and enable the patient to recover and survive.
 | Volunteers offered £3,500 to be infected with coronavirus A new scientific study is asking members of the public to volunteer to be infected with a coronavirus in return for cash. |
 | Behind the scenes: Scientists prepare for COVID-19 vaccine test A team of scientists jostled for a view of the lab dish, staring impatiently for the first clue that an experimental vaccine against the new ... |
 | Breakthrough for the COVID-19 vaccine in poultry, adaptable to humans Researchers at the Miguel Institute, supported by the Ministry of Science and Technology, explain the research at a press conference. They claim the vaccine’s efficacy ... |
In topics
Recommended
 World
World
India reports 9 Pakistani Aircraft Destroyed In Operation Sindoor Strikes
 World
World
Thailand Positions Itself As a Global Wellness Destination
 World
World
Indonesia Accelerates Procedures to Join OECD
 World
World
South Korea elects Lee Jae-myung president
Popular article
 World
World
22nd Shangri-La Dialogue: Japan, Philippines boost defence cooperation
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World