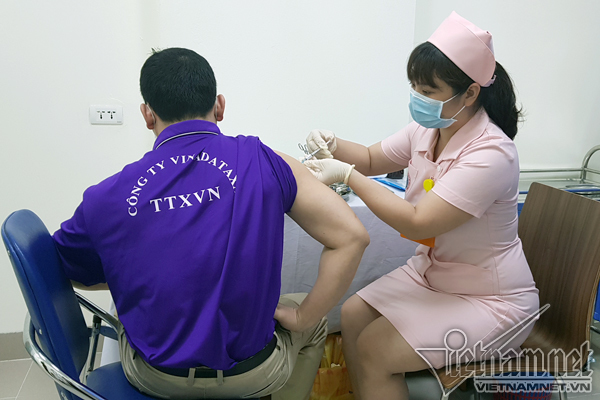
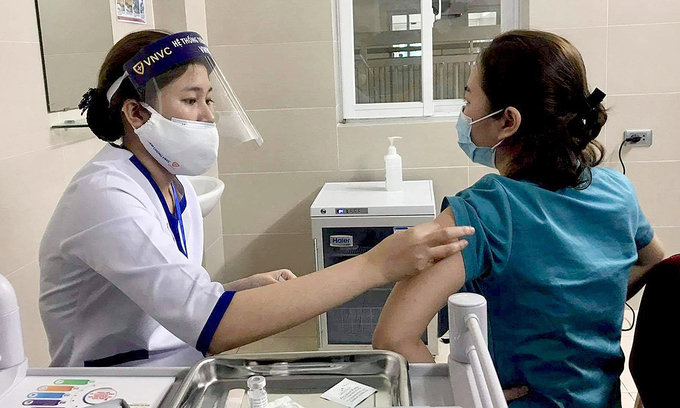
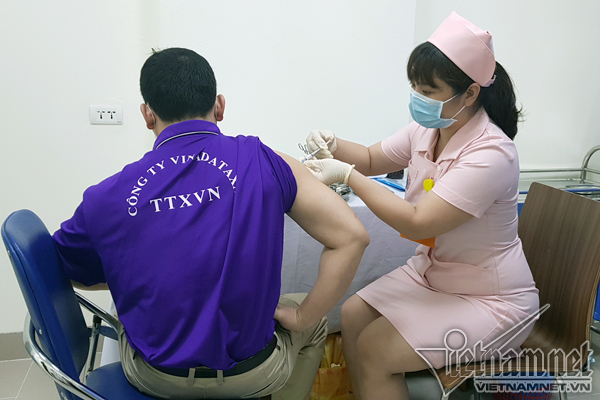
Vietnam kicks start second trial jabs of homegrown Covivac vaccine
 | |
|
According to VNE, the 6 volunteers first in line for the second jab are also the first group to get the first injection back on March 15. As they are all in normal health condition, they are eligible for the follow-up injection.
The 120 volunteers aged 18-59 are divided into 5 groups who received different doses (1 mcg, 3 mcg, 10 mcg, 1 mcg with supplemented adjuvant and placebo). All the second jabs of phase 1 of the clinical trial are expected to complete by May 15, according to Associate Professor, Dr. Vu Dinh Them, Director of the Center for Clinical Trial, Central Institute of Hygiene and Epidemiology. Reports evaluating the vaccine’s immunological efficacy will be published two weeks later before Covivac enters phase 2 of human trials in June.
“If the research process goes smoothly, Covivac vaccine is expected to complete all three phases of the clinical trials by the end of 2022”, Thiem was quoted by VNE.
To date, all volunteers only experienced mild reactions post-injection, including pain at the injection site, headache, muscle pain, etc. The symptoms wore off within one or two days. No severe reactions are reported.
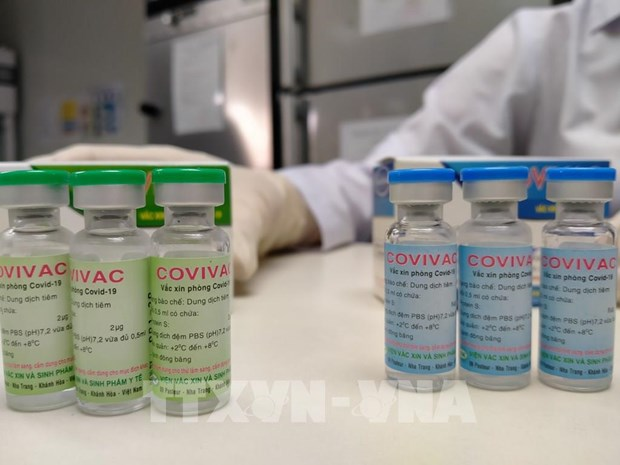
Covivac is Vietnam’s second indigenous Covid-19 vaccine to enter human trial. It was studied and developed by the Institute of Vaccines and Medical Biologicals (IVAC). One shot of Covivac is proved effective on the British and South African variants of nCoV, which have emerged several months ago and are more dangerous than the original variant.
IVAC earlier announced one dose of Covivac is priced at VND 60,000 (US $2.6). The Institute has a production capacity of 6 million doses per year. The number could be raised to 30 million doses per year, it added.
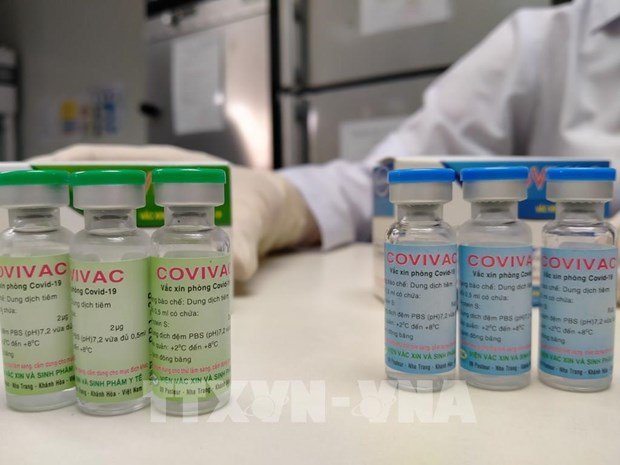 |
| One dose of Covivac is priced at VND 60,000 (US $2.6) (Photo: VNA) |
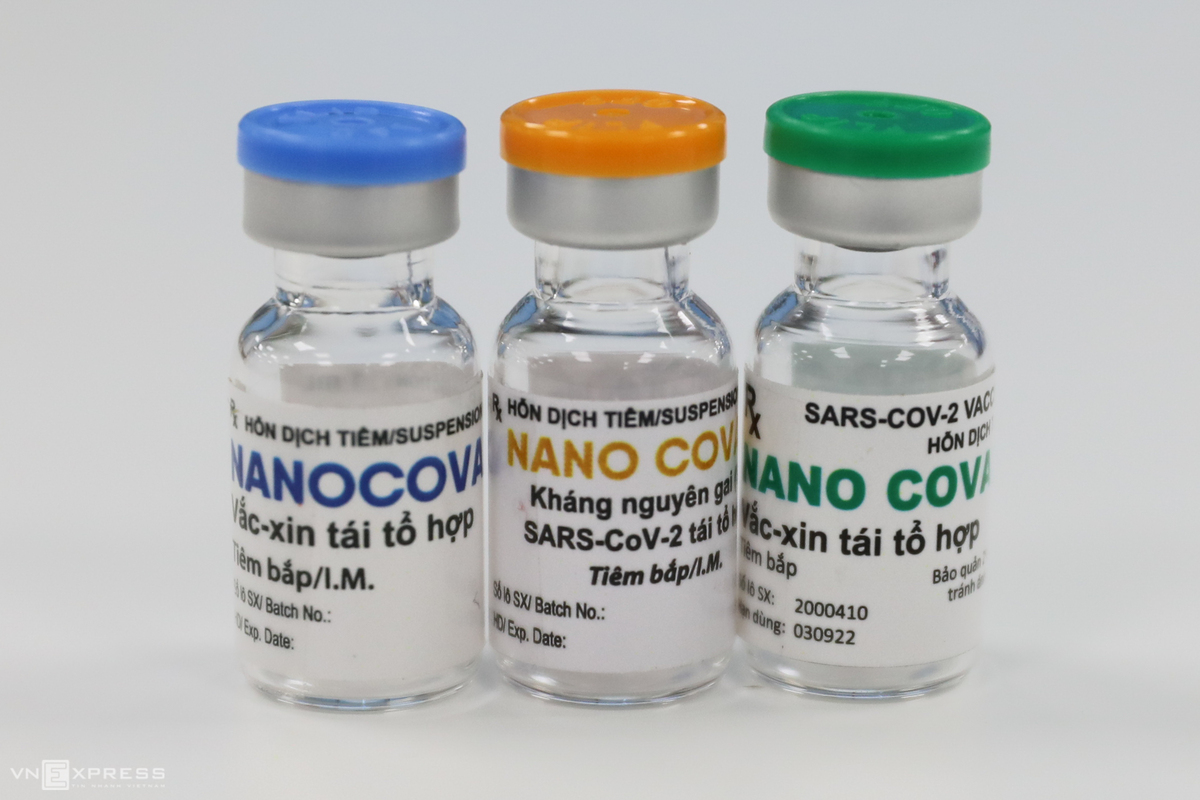
Meanwhile, Nanocovax - the production of Nanogen BioPharmerticeul company – is set to enter phase 3 of the human trial this May. Accordingly, Nanogen originally planned to start the last phase in August and end on February 22. It involves 1,500 – 3,000 volunteers aged 12-75 to further evaluate the immunogenicity and protectiveness of the vaccine. However, the first two phases yield promising results and go smoothly, prompting researchers to speed up the trials.
Nanogen and researchers from the Military Medical Academy (MMA) are urgently deploying plans for the third phase, making sure everything is in line with protocols, according to Prof. Dr. Do Quyet, Director of MMA. MMA is expecting to submit the second phase’s report to the Health Ministry and the National Biomedical Research Ethics Council. Among the three doses under trial, (25 mcg, 50 mcg, and 75 mcg), one yields the highest immunogenicity will be chosen for the third phase.
Nanocovax is the first made-in-Vietnam COVID-19 vaccine to enter human trials. The health ministry earlier has assessed Nanogen’s Covid-19 vaccine candidate among the most promising, having been successfully produced on a laboratory scale and provoked immunogenicity during animal testing.
“Each injection is priced at around VND 120,000 (US $5.18). Nanocovax has two injections, it will cost each individual a sum of VND 240,000 for the COVID-19 vaccination”, Dr. Do Minh Si, Nanogen’s research and development director said on December 10, adding that the price was based on production costs, clinical trials costs and other relevant fees.
Nanogen’s current production capacity is around 10-20 million doses per year. The company is planning to upgrade its factory to increase the production scale to 50-70 million doses per year. The company said it would prioritize domestic distribution before exporting the vaccine.
Besides, Vietnam is having two other potential candidates studied and produced by Vabiotech, Polyvac.
| Vietnam recorded 10 new cases of COVID-19 between 6 am and 6 pm April 20. All the 10 new patients, one Indian and nine Vietnamese arrived from abroad and were immediately put into quarantine after arrival. From 6 pm on April 20 to 6 am on April 21, Vietnam did not report any new cases, while an additional 14,386 people were vaccinated against COVID-19 on April 20. |
 | COVID-19 vaccine trials: Six volunteers get second shots of Covivac Six volunteers joining the human trials of Covivac on April 12 received their second shots of the homegrown COVID-19 vaccine. |
 | Vietnam to have second Covid-19 vaccine available in early 2022 Vietnam’s homegrown Covid-19 vaccine – Covivac – is expected to be ready for use in Quarter 1 of 2022 after going through all needed procedures. |
 | Vietnam to launch first homegrown COVID-19 vaccine in September Vietnam expects to introduce its first domestically- developed COVID-19 vaccine in September. |
Recommended
 National
National
Vietnam News Today (Jun. 2): Vietnamese Trade Mission Sounds Out Business Opportunities in United States
 National
National
Vietnam News Today (Jun. 1): Vietnamese, Japanese Firms Foster Partnership
 National
National
Vietnam News Today (May 31): Vietnam Strongly Supports Laos’s National Development
 National
National
Vietnam News Today (May 30): Vietnam, Venezuela Reinforce Ties Through People-to-people Diplomacy
 National
National
Vietnam News Today (May 29): Vietnam and Hungary to Expand Cooperation into New Areas
 National
National
Vietnam News Today (May 28): Vietnam and China Discuss Strategic Cooperation Orientations
 National
National
Vietnam News Today (May 27): Vietnam Treasures Multifaceted Collaboration with France
 National
National