Vietnam to have second Covid-19 vaccine available in early 2022
 | |
|
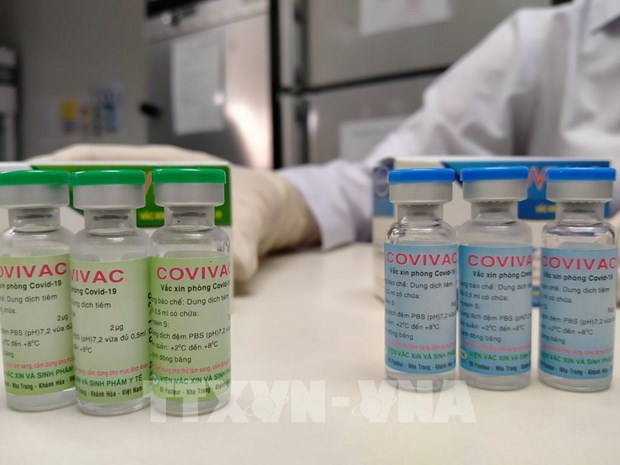
“We are accelerating the speed of research and human trials. If things go as planned, Covivac vaccine would be available for use in Vietnam in the first quarter of 2022. It’s possible that we could even export the vaccine”, Mr. Duong Huu Thai, director of the Institute of Vaccines and Medical Biologicals (IVAC), said on April 11.
According to the director, IVAC has production capacity of 6 million doses of Covivac vaccine a year. The number could be soon raised to 30 doses per year. In addition to the reasonable price (VND 60,000 – US $2.6), Covivac is easily stored at refrigerator temperature which facilitates the transportation process.
The Covivac vaccine has similarities with the imported AstraZeneca vaccine. Both use vector technology. However, their cost of use is different. COVIVAC cultures chicken eggs with embryo technology that IVAC has used to produce seasonal flu vaccines for many years, while AstraZeneca is produced by cell culture technology.
Covivac is the research work of IVAC under the Health Ministry. It had earlier yielded safe results and strong immunity responses on mice, rabbits, etc. Given promising results on animals, the vaccine is granted to enter human trials starting January, two months earlier than expected. The first phase of Covivac’s human trials, which takes place at Hanoi Medical University, involves 120 volunteers who are divided into 5 groups of 12-15 people. Each group receives a different dose of vaccine (1 mcg, 3 mcg, 10 mcg, and placebo).
All volunteers will get two jabs of the made-in-Vietnam vaccine, the second is 28 days after the first one. They will have their health monitored throughout the researching process.
“To date, 66 volunteers have been given the first jab. It is expected that the first leg of Covivac’s human trials will be completed in the next one or two weeks”, Thai was quoted as saying. “All of the vaccinated ones are currently in stable health, some experienced mild post-injection reactions, but the symptoms wore off within one day”.
If reports in July show the vaccine meets all safety standards, capable of generating antibodies and granted approval from the Ministry of Health, Covivac will enter the second human trial phase with 300 volunteers in Thai Binh provinces.
Meanwhile, Nanogen’s NanoCovax is also preparing for phase 3 of its clinical trials this May, involving 10,000-15,000 people in both Vietnam and other countries in Asia with severe coronavirus outbreaks. Test results of the second trial are set to be available in late April. NanoCovax has so far proven safe and generated a high amount of antibodies that are effective against new variants of the novel coronavirus.
 | |
|
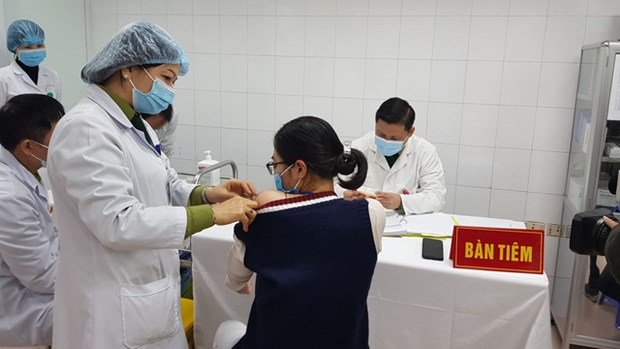
NanoCovax, known as Vietnam’s first domestically-developed COVID-19 vaccine, is expected to be introduced for use this September, according to the National Steering Committee for COVID-19 Prevention and Control on March 22.
NanoCovax, developed by the Nanogen Pharmaceutical Biotechnology JSC, is the first COVID-19 vaccine of Vietnam to be tested in clinical trials.
| Vietnam recorded another imported COVID-19 case from 6 am to 6 pm on April 11, raising the total number of infections to 2,693, said the Ministry of Health. The new patient is a 27-year-old man who has been quarantined since his entry via Ha Tien international border gate in the Mekong Delta province of Kien Giang on April 8. The April 12 morning news of the Ministry of Health confirmed 3 additional imported cases of COVID-19 recorded in Hanoi and Thai Nguyen. All of them were immediately isolated and treated. |
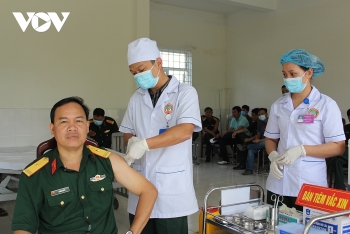 | Frontline officers, soldiers in central Vietnam get prioritized Covid-19 vaccines 60 officers and soldiers in Quang Nam province’s Armed Force are given the first dose of AstraZeneca vaccine on April 9. |
 | Vietnam COVID-19 Updates (April 10): Vietnam to start mass vaccine production in August Vietnam could start mass production of its own Covid-19 vaccine in August if a three-phase human trial is completed in May. |
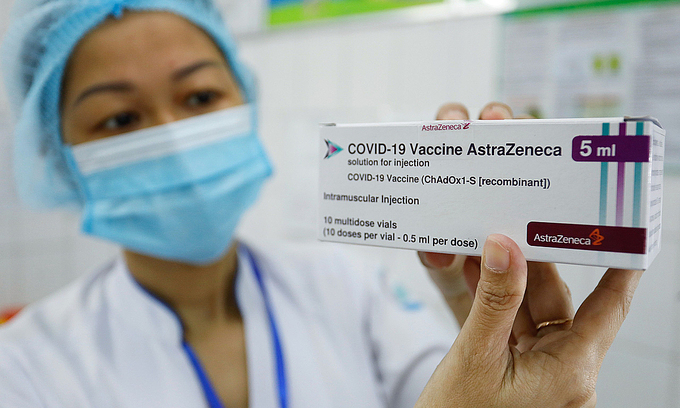 | Over 55,000 Vietnamese receiving AstraZeneca vaccine, low rate of post-injection reaction shown Since the AstraZeneca vaccination campaign was launched in Vietnam on March 8, there have been 33% of recipients displaying commonly mild reactions |
In topics
Recommended
 National
National
Vietnam News Today (Jun. 7): Prime Minister works with Estonian firms to accelerate projects in Vietnam
 National
National
Vietnam News Today (Jun. 6): Foreign Investment in Vietnam Surges in Five Months
 National
National
Vietnam News Today (Jun. 5): PM sets off for attendance at UNOC 3 in France, official visits to Estonia, Sweden
 National
National
Vietnam News Today (Jun. 4): Vietnam - Promising Candidate for Southeast Asia’s Next Powerhouse
Popular article
 National
National
Shangri-La Dialogue 22: Vietnam Highlights Some Issues of Ensuring Stability in a Competitive World
 National
National
Vietnam News Today (Jun. 3): PM Pham Minh Chinh to Attend UN Ocean Conference, Visit Estonia, Sweden
 National
National
Vietnam News Today (Jun. 2): Vietnamese Trade Mission Sounds Out Business Opportunities in United States
 National
National