Vietnam’s COVID-19 Nanocovax vaccine to be priced US $10.3 per dose
| Vietnam’s COVID-19 vaccine to be priced under US $22 | |
| Lastest COVID-19 vaccine updates, global experts are optimistic about vaccine development |
| Lastest COVID-19 vaccine updates: FDA says Pfizer-BioNTech safe, effective, China’s Sinovac vaccine is 97 percent effective |
 |
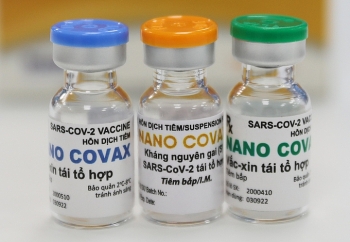
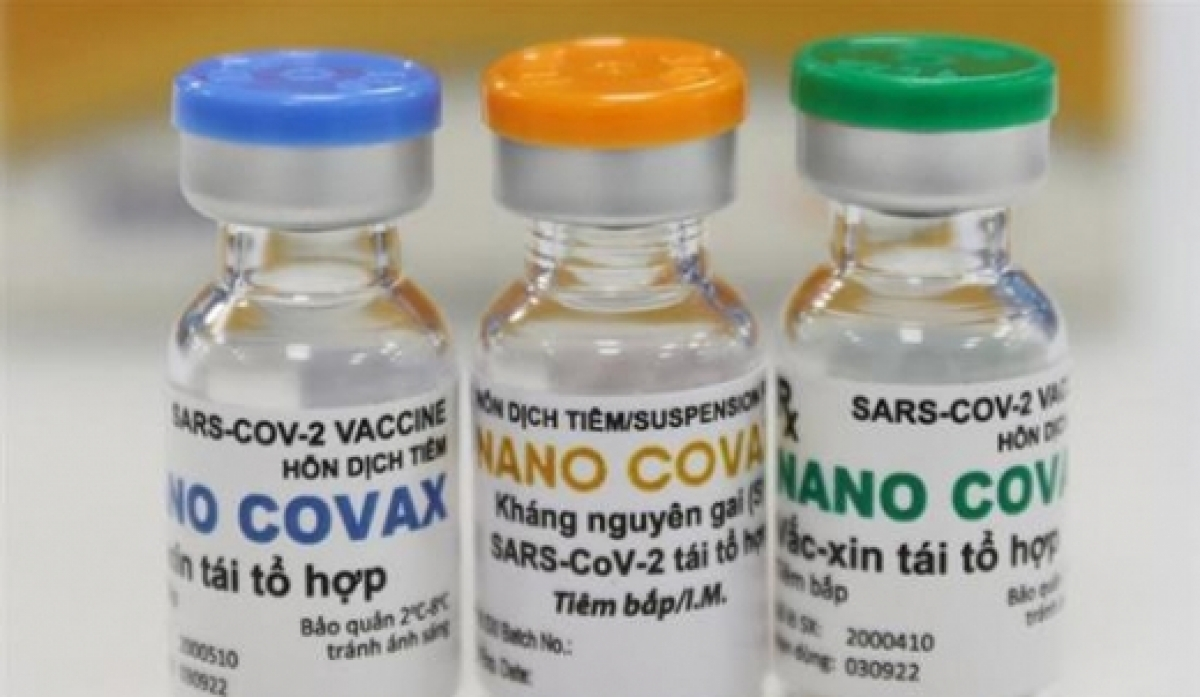
| Nanocovax is priced at only $10.3 per dose (Photo: VNE) |
“Each injection is priced at around VND 120,000 (US $5.18). Nanocovax has two injections, it will cost each individual a sum of VND 240,000 for the COVID-19 vaccination”, Dr. Do Minh Si, Nanogen’s research and development director said on December 10, adding that the price was based on production costs, clinical trials costs and other relevant fees.
Notably, the Director said he was targeting Nanocovax to be over 90 percent effective, “like other potential COVID-19 vaccine candidates in the world”, Dan Tri reported.
Earlier on December 8, a representative from Nanogen company stated that the company was planning to priced Nanocovax at no more than VND 500,000 (US $21.62). The company was also trying to include the vaccine into the list of drugs covered by health insurance.
Nanogen’s current production capacity is around 10-20 million doses per year. The company is planning to upgrade its factory to increase the production scale to 50-70 million doses per year.
“We will prioritize domestic distribution before any plan to export the vaccine”, Si emphasized.
 |
| Dr. Do Minh Si, Nanogen’s research and development director (Photo: Dan Tri) |
Nanocovax is the first made-in-Vietnam COVID-19 vaccine to enter human trials. Nanogen company is running the first phase of human trials on 60 volunteers starting December 10 before moving to the second phase with some 400 volunteers. The third stage of the clinical trials is yet to be decided.
If everything goes well with the clinical trials, Nanocovax, which developed under recombinant protein technology, is expected to enter mass vaccination in May, 2021.
“If there were no vaccine as soon as possible, we’ll have to wait until the latter half of 2021, or even 2022 to put the epidemic under control. It’s like being prisoned if we lose a few years of freedom”, Ho Nhan, General Director of Nanogen company was quoted by VNE as saying.
 |
| NanoCovax (Photo: Dan Tri) |
 |
| First Vietnamese girl signing for voluntary form for the phase one of Nanocovax's human trials (Photo: Dan Tri) |
 |
| Registration desk (Photo: Dan Tri) |
Nanogen’s COVID-19 vaccine is evaluated as safe. Side effects on mice and monkeys are “negligible”, only cause mild irritation and itching which last for only 30 minutes. Anatomy of vaccinated mice found no internal organ damages.
The health ministry earlier has assessed Nanogen’s Covid-19 vaccine candidate among the most promising, having been successfully produced on a laboratory scale and provoked immunogenicity during animal testing.
| Vietnam has four Covid-19 vaccines produced by Nanogen, Vabiotech, Polyvac and the Institute of Vaccines and Medical Biologicals (IVAC) currently under research. Vabiotech, Polyvac are currently evaluating their vaccines on animals, having completed the laboratory-scale production process. Meanwhile, IVAC will continue to cooperate with Russia and “actively contact with China to have access to China’s vaccine”. |
| On global scale, there are currently 11 COVID-19 vaccine candidates under the third phase of human trials. Pfizer/BioNTech’s vaccine (the US) is the first vaccine to complete the trials with 95 percent effectiveness and granted the emergency use authorization from the UK and Bahrain. Meanwhile, Moderna’s vaccine is on its final clinical trial phase, with effective rate reaches 94.5 percent. Oxford/ AstraZeneca is 70-90 percent effective, depending on the injection dose. Russia’s Sputnik V (95 percent effective) is scheduled to begin mass vaccination next week. Moderna’s vaccine is priced at 37 USD per dose, meanwhile, Pfizer’s vaccine and Oxford’s vaccine are more reasonably priced at 19 USD and 3 UDD per dose, respectively. |
 | India refutes reports it declined emergency approval for AstraZeneca's Covid-19 vaccine India's health ministry denied reports saying that the country's drugs regulator had refused emergency authorization for the AstraZeneca Covid-19 vaccine candidate and one developed locally ... |
 | COVID-19 Updates (Dec. 10): Vietnam COVID-19 vaccine trial process officially launched The Military Medical Academy officially launched the Covid-19 vaccine clinical trial program and announced the recruitment of volunteers to participate in the research. |
 | Vietnam's COVID-19 vaccine trial: Requirements for volunteers In order to be selected for the COVID-19 trial process, volunteers are demaded to meet some requirements including health standard, age from 18 to 60, ... |
Recommended
 National
National
Vietnam News Today (May 31): Vietnam Strongly Supports Laos’s National Development
 National
National
Vietnam News Today (May 30): Vietnam, Venezuela Reinforce Ties Through People-to-people Diplomacy
 National
National
Vietnam News Today (May 29): Vietnam and Hungary to Expand Cooperation into New Areas
 National
National
Vietnam News Today (May 28): Vietnam and China Discuss Strategic Cooperation Orientations
 National
National
Vietnam News Today (May 27): Vietnam Treasures Multifaceted Collaboration with France
 National
National
Vietnam Commits to Building an Inclusive, Sustainable and Cohesive ASEAN
 National
National
Vietnam Proposes Vision for Responsible Digital Journalism Cooperation
 National
National