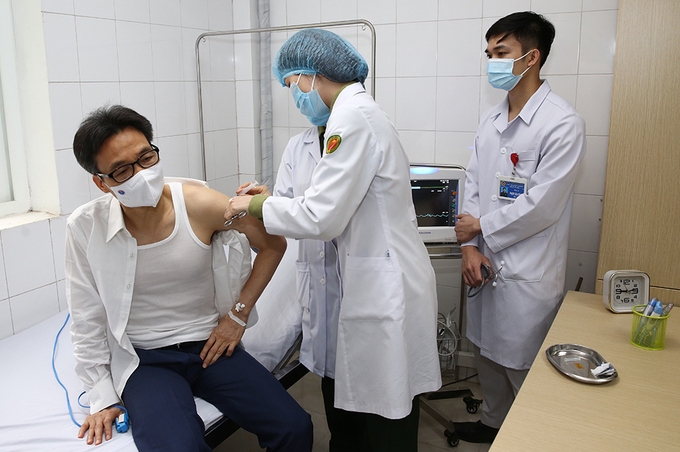
Deputy PM visits Vietnam’s first COVID-19 vaccine volunteers
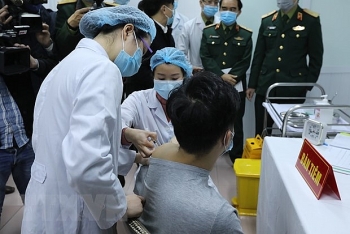 | Made-in-Vietnam COVID-19 vaccine promises a bright prospect |
 | Vietnam all geared up for COVID-19 vaccine trials |
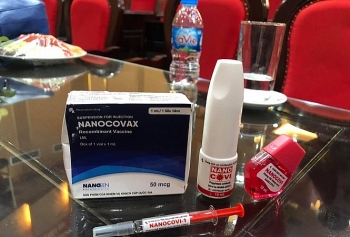 | Vietnam to roll out nasal spray and eye-drop COVID-19 vaccine |
 |
| Deputy Prime Minister Vũ Đức Đam visits the volunteers on December 20 (Photo: VNA) |
The initial results of the vaccine, which indicates the vaccine is safe for human, were reported to Deputy Prime Minister Vu Duc Dam upon his visit.
As no abnormal reaction has been found on the volunteers, one man and two women aged 20-25, on December 21, the Military Medical Academy is scheduled to administer the vaccine on the remaining volunteers who registered for the first phase of the trial. According to leaders of the academy, after reviewing records of the vaccine’s effects on mouse and monkey, experts hope that following the second and third phases, the immunogenesis of the vaccine will be fully assessed and meet the requirements.
The trial will have three phases. In the first, the vaccine will be injected into 60 persons who are divided into three groups to receive different doses of 25 mcg, 50mcg, and 75mcg.
The objective of the first phase is to evaluate the safety of the vaccine. Following the success of the first phase, the second will involve 400-600 people to define the optimal dose, and the third will see the engagement of at least 1,500-3,000 people. The figure may be expanded to 10,000-30,000.
After the third phase, the vaccine’s safety, immunogenesis and effectiveness are expected to be assessed.
The Ministry of Health has created all favorable conditions to shorten the time for the trial phases compared to the normal conditions but still ensuring all steps, safety and scientific requirements.
Deputy Prime Minister Dam spoke highly of the efforts of the Ministry of Health, the Ministry of Science and Technology, and the Military Medical Academy as well as the research and production units of the vaccine.
He said that the implementation of the vaccine’s trial phases must be continued with the full observation of regulations of the Ministry of Health in a safe and prompt manner.
“If the trial is successful, it would be not only a pride of the health science and healthcare sector but also an effective tool to fight the pandemic,” stated the Deputy PM.
Citing experience from the production of medical bio-products for SARS-CoV-2 testing, the Deputy PM asked the research and production units as well as State management agencies to work closely with one another to “race against the time”, while continue preparing necessary conditions for the next steps following the success of the vaccine trials, with calculation for both good and bad circumstances.
| In the context of high prices and limited supply of the vaccines against the pandemic in the world, the acceleration of the research and production of the vaccine inside the country is necessary, said a leader of the Ministry of Health. However, Deputy PM Dam underlined that vaccination is still a future matter, highlighting the need to focus on preventive measures against the pandemic. He asked the Ministry of Science and Technology, the Military Medical Academy, and the Bio-Technology Institute to build and develop a centre for the research and development of vaccines against newly-emerging pandemics, including the construction of a bio-safety lab at at least Level Three, thus helping the country become ready for new pandemics and disasters related to human’s health in the future. He also agreed with the proposal of the Military Medical Academy on the increase of training for response against emergency cases and disasters in schools and medical training facilities. |
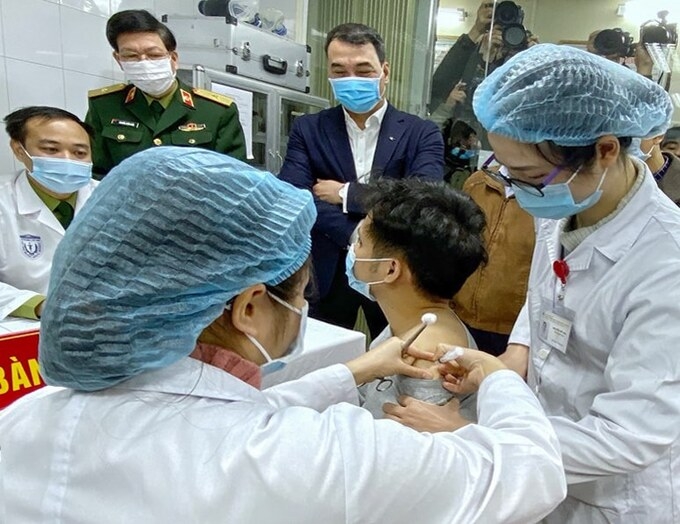 |
| A man is injected with Nanocovax, the first made-in-Vietnam Covid-19 vaccine, in Hanoi, December 17, 2020 (Phôt: VNE) |
Human trials of Nanocovax, the first Vietnamese Covid-19 vaccine, began on December 17 morning with the first three subjects getting shots of it.
On December 22 morning, another 17 volunteers are also injected with the jabs at Hanoi Military Medical University. The volunteers are kept under watching for another 72 hours to detect if there are any further complications.
Thus, the first 20 volunteers among 60 in the first phase has got the first injection (dose of 25 mcg). The remaining 40 volunteers will be divided into two other groups to respectively recieve 50 mcg, and 75 mcg of the vaccine, VNExpress exported.
In the second phase, the vaccine will be injected into 400-600 volunteers aged from 12 to 75 to determine the optimal dose. The third phase will be carried out on at least 1,500 to 3,000 volunteers and even up to 10,000 to 30,000 volunteers. This phase aims to evaluate the safety, immunity, and efficacy of the vaccine.
Nanocovax is the fist domestically-produced COVID-19 vaccine in Vietnam. It is priced at VND120,000 ($5.17) per dose, scheduled to enter mass production in May 2021.
Nanogen’s COVID-19 vaccine is evaluated as safe. Side effects on mice and monkeys are “negligible”, only cause mild irritation and itching which last for only 30 minutes. Anatomy of vaccinated mice found no internal organ damages.
The health ministry earlier has assessed Nanogen’s Covid-19 vaccine candidate among the most promising, having been successfully produced on a laboratory scale and provoked immunogenicity during animal testing.
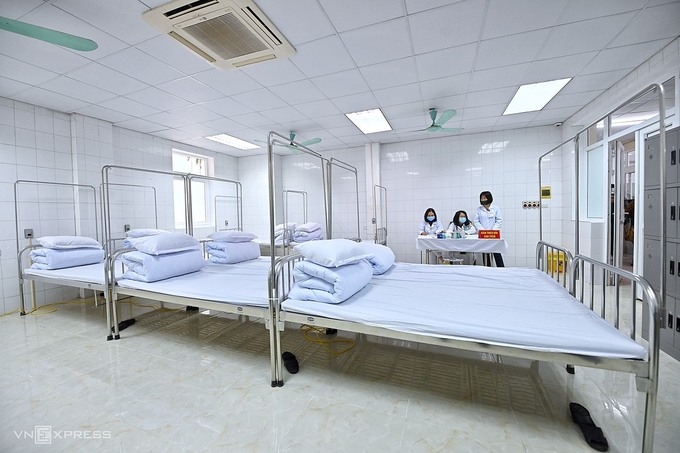 |
| Volunteers who have got the jabs will be further monitored in this room at Hanoi Military Medical University (Photo: VNE) |
| Vietnam has four Covid-19 vaccines produced by Nanogen, Vabiotech, Polyvac and the Institute of Vaccines and Medical Biologicals (IVAC) currently under research. Vabiotech, Polyvac are currently evaluating their vaccines on animals, having completed the laboratory-scale production process. Meanwhile, IVAC will continue to cooperate with Russia and “actively contact with China to have access to China’s vaccine”. |
 | PM: Vietnam creates maximum conditions for Covid-19 vaccine production Prime Minister Nguyen Xuan Phuc on December 21 requested relevant agencies to create maximum conditions for domestic units to carry out Covid-19 vaccine research and ... |
 | World breaking news today (December 22): Joe Biden receives Covid vaccine on live television World breaking news today (December 22): Joe Biden receives Covid vaccine on live television. Meanwhile, Pompeo blames Russians for massive cyberattack, EU approves Pfizer's COVID-19 ... |
 | PM Phuc encourages establishment of Vietnam's vaccine research and development center Prime Minister Nguyen Xuan Phuc on Monday tasked the Military Medicine Academy to work with relevant institutions and agencies to draft a vaccine research and ... |
Recommended
 National
National
Vietnam News Today (Jun. 2): Vietnamese Trade Mission Sounds Out Business Opportunities in United States
 National
National
Vietnam News Today (Jun. 1): Vietnamese, Japanese Firms Foster Partnership
 National
National
Vietnam News Today (May 31): Vietnam Strongly Supports Laos’s National Development
 National
National
Vietnam News Today (May 30): Vietnam, Venezuela Reinforce Ties Through People-to-people Diplomacy
Popular article
 National
National
Vietnam News Today (May 29): Vietnam and Hungary to Expand Cooperation into New Areas
 National
National
Vietnam News Today (May 28): Vietnam and China Discuss Strategic Cooperation Orientations
 National
National
Vietnam News Today (May 27): Vietnam Treasures Multifaceted Collaboration with France
 National
National