Vietnam all geared up for COVID-19 vaccine trials
 |
| Employees of Nanogen Pharmaceutical Biotechnology JSC work in a molecular biology room to produce made-in-Vietnam vaccine to be tested on human, December 2020. (Photo: VNE) |
Nguyen Ngo Quang, deputy head of the Administration of Science, Technology and Training under the Ministry of Health, confirmed around 200 people had signed up within a week to be vaccinated with Nanocovax, a Covid-19 vaccine developed by Nanogen Pharmaceutical Biotechnology JSC, VNE reported.
Vietnam Military Medical Academy, as part of first phase trials, will oversee administration of the Covid-19 vaccine.
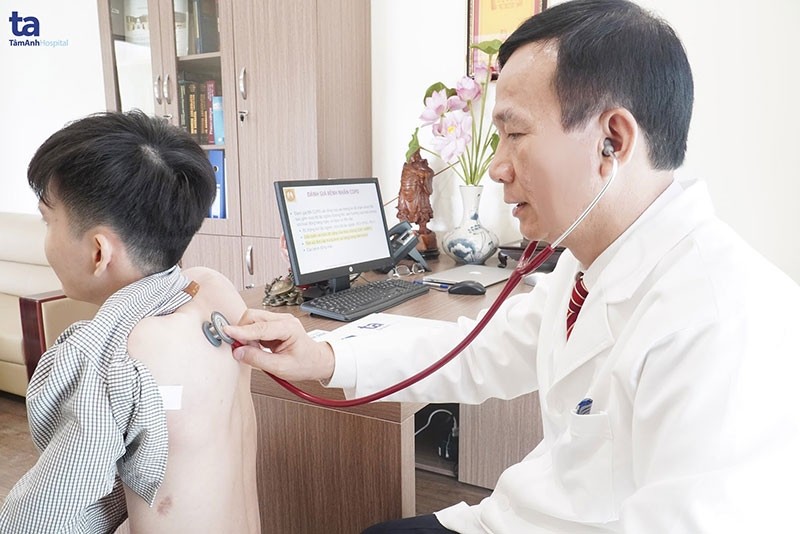
The academy is racing against time to screen volunteers eligible for the first human trial stage. Selected candidates are between 18 and 50 of age.
They will receive two intramuscular injections of the vaccine or a placebo, with an interval of 28 days between each jab. Each volunteer will have their health monitored for 56 days to assess the efficiency of the vaccine and continue to be observed for sixth months following administration.
Do Minh Sy, Nanogen's director for research and development, said all risks and variables have been calculated, with medical staff from 103 Military Hospital and the Vietnam National Institute of Burns on standby.
Nanogen has contracted an insurance company to cover the volunteers, with certain banks agreeing to compensate those who fail to obtain coverage with up to VND20 billion ($863,290), he added.
 | |
|
Nanocovax is expected to be priced at VND120,000 ($5.17) per dose.
Along with injections, Vietnam’s COVID-19 Nanocovax vaccine will also be developed in the form of eye-drop and nasal spray for special subjects.
“Nanocovax is way more effective and generates faster immunity than other made-in-Vietnam candidates currently developed”, Nhan was quoted by Tien Phong as saying. “However, there are still people unqualified for normal injection. Thus, we have come up with the nasal spray and eye-drop vaccination plan to make sure 100 percent of Vietnamese are vaccinated against the deadly coronavirus”.
Nanocovax is scheduled to enter mass production in May 2021.
 |
| A staff producing Nanocovax vaccine (Photo: VNE) |
 |
| (Photo: VNE) |
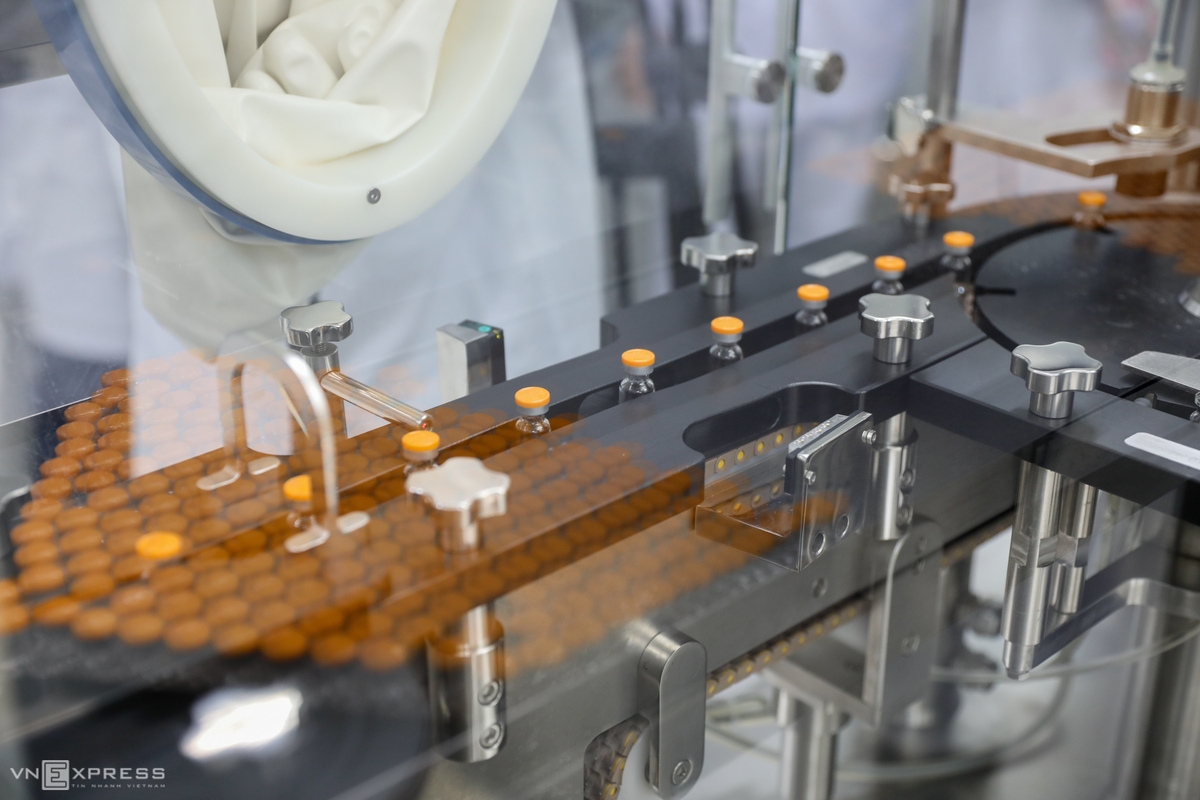
Nanogen’s current production capacity is around 10-20 million doses per year. The company is planning to upgrade its factory to increase the production scale to 50-70 million doses per year.
Nanogen’s COVID-19 vaccine is evaluated as safe. Side effects on mice and monkeys are “negligible”, only cause mild irritation and itching which last for only 30 minutes. Anatomy of vaccinated mice found no internal organ damages.
The health ministry earlier has assessed Nanogen’s Covid-19 vaccine candidate among the most promising, having been successfully produced on a laboratory scale and provoked immunogenicity during animal testing.
 | |
|
| Vietnam has four Covid-19 vaccines produced by Nanogen, Vabiotech, Polyvac and the Institute of Vaccines and Medical Biologicals (IVAC) currently under research. Vabiotech, Polyvac are currently evaluating their vaccines on animals, having completed the laboratory-scale production process. Meanwhile, IVAC will continue to cooperate with Russia and “actively contact with China to have access to China’s vaccine”. |
| On global scale, there are currently 11 COVID-19 vaccine candidates under the third phase of human trials. Pfizer/BioNTech’s vaccine (the US) is the first vaccine to complete the trials with 95 percent effectiveness and granted the emergency use authorization from the UK and Bahrain. Meanwhile, Moderna’s vaccine is on its final clinical trial phase, with effective rate reaches 94.5 percent. Oxford/ AstraZeneca is 70-90 percent effective, depending on the injection dose. Russia’s Sputnik V (95 percent effective) is scheduled to begin mass vaccination next week. Moderna’s vaccine is priced at 37 USD per dose, meanwhile, Pfizer’s vaccine and Oxford’s vaccine are more reasonably priced at 19 USD and 3 UDD per dose, respectively. |
 | US’ Pfizer Covid-19 vaccine distributed to hospitals for human injection from December 14 Today (Dec 14), the very first Covid-19 vaccine in the US, Pfizer-BioNTech will be rolled out to hospitals and injected into millions of vulnerable people ... |
 | Vietnam to roll out nasal spray and eye-drop COVID-19 vaccine Along with injections, Vietnam’s COVID-19 Nanocovax vaccine will also be developed in the form of eye-drop and nasal spray for special subjects. |
 | Vietnam to conduct COVID-19 vaccine trial on willing volunteers Vietnam is currently recruiting willing volunteers for phase 1 COVID-19 vaccine clinical trial, with the injection is slated to be conducted starting December 17. |
Recommended
 National
National
Vietnam News Today (May 23): Vietnam–France Comprehensive Strategic Partnership Opens New Horizons for Cooperation
 National
National
Vietnam News Today (May 22): Stronger Vietnam-Israel Cooperation Expected in Science, Innovation and Labor
 National
National
Vietnam News Today (May 21): Vietnam Attends UN Commission on Crime Prevention and Criminal Justice's 34th Session
 National
National
Deep Affection of International Friends
Popular article
 National
National
Vietnam News Today (May 20): Hanoi Named Top Cultural, Artistic Destination in Asia
 National
National
Vietnam News Today (May 19): Norway Hails Vietnam’s Continued Emphasis on Upholding International Law
 National
National
Vietnam News Today (May 18): Cannes 2025: Vietnam Rising as New Destination for International Filmmakers
 National
National