How does Oxford- AstraZeneca - first approved COVID-19 vaccine in Vietnam - work?
 |
| Oxford- AstraZeneca was pronounced 90 percent effective against the novel coronavirus (Photo: BioWorld) |
AstraZeneca-Oxford vaccine's efficacy
Oxford- AstraZeneca was pronounced 90 percent effective in preventing nCoV contraction, with no serious side effects reported, Oxford university and its partner AstraZeneca said in late November.
How does the AstraZeneca-Oxford vaccine work?
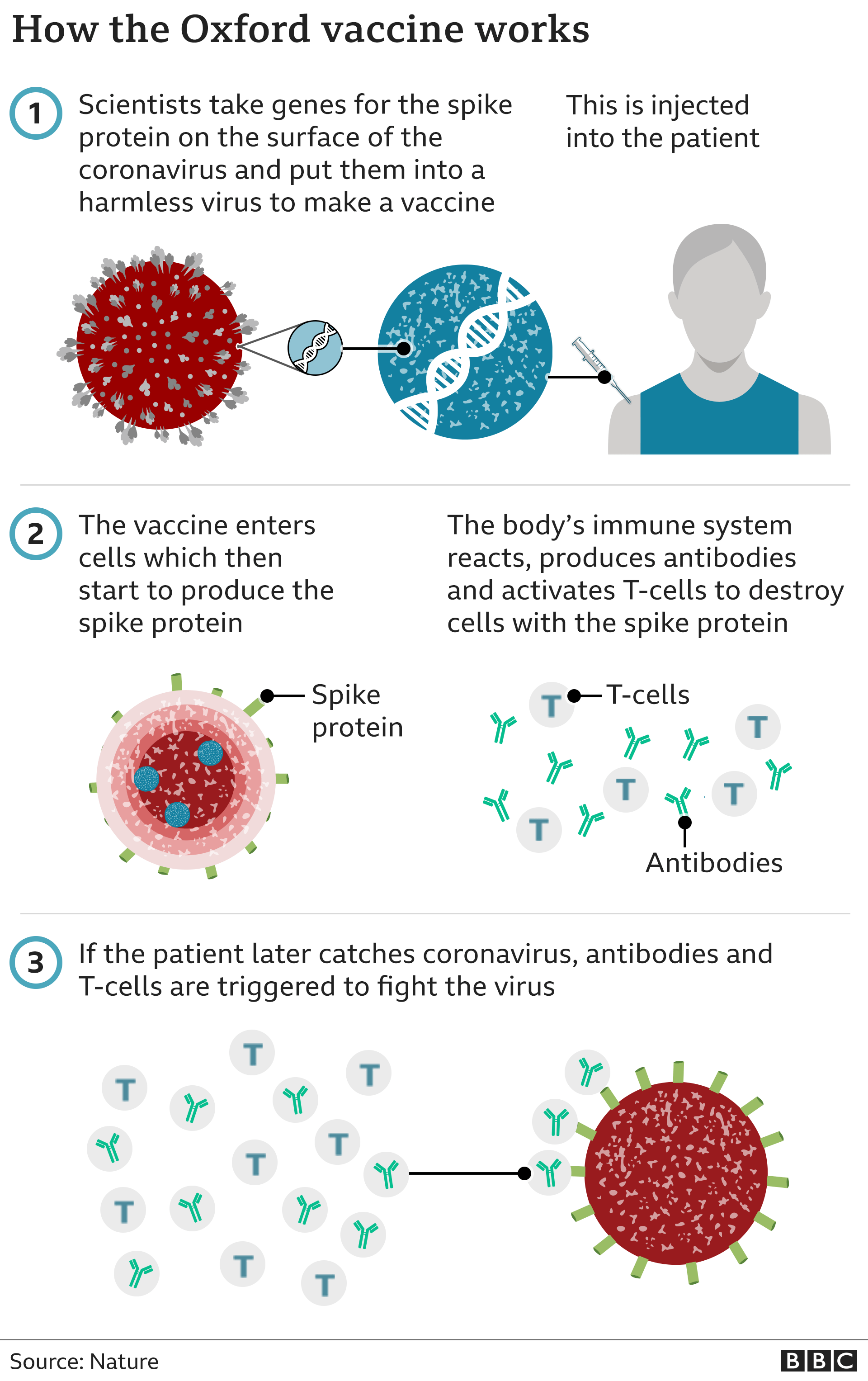
The vaccine – called ChAdOx1 nCoV-19 – uses a harmless, weakened version of a common virus that causes a cold in chimpanzees, according to the Irish Examiner. The virus is genetically modified so that it is impossible for it to grow in humans.
Scientists have transferred the genetic instructions for coronavirus’s specific “spike protein” – which it needs to invade cells – to the vaccine.
When the vaccine enters cells inside the body, it uses this genetic code to produce the surface spike protein of the coronavirus.
This induces an immune response, priming the immune system to attack coronavirus if it infects the body.
 |
| Illustrative photo: Nature/ via BBC |
When was the vaccine approved?
The vaccine was approved for use by the MHRA (the UK's regulatory body) on 30 December
It was developed quickly because Oxford University researchers had already done a lot of work on developing a vaccine which could be adapted to tackle different diseases.
Hoppitals administered the first doses to older patients early in January 2021. Supplies were then sent to hundreds of GP-led services and care homes across the UK.
The MHRA approved the use of two full doses, which was found to be 62% effective.
And as with the other vaccines, scientists don't yet know if it stops people catching Covid - that's something they won't know until they can see the impact of vaccination over time, according to BBC.
How long does it protect against Covid for?
As with all the vaccines being developed against coronavirus, we don't know yet.
It may be that people need annual vaccinations, as happens with the flu jab.
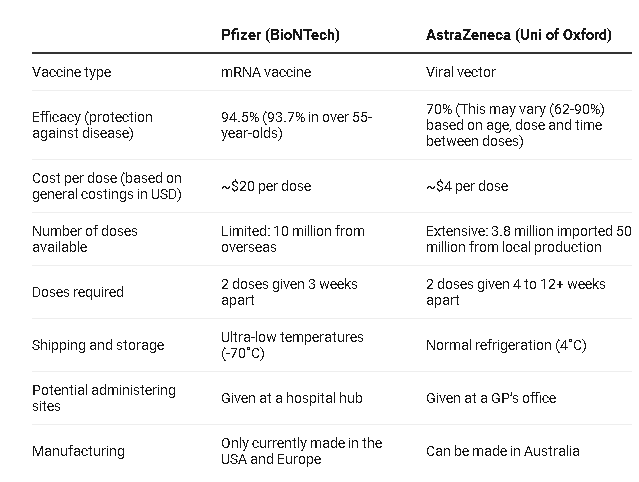
Advantages of Oxford- AstraZeneca vaccine over other candidates
With affordable and favorable storing conditions, the UK’s Oxford – AstraZeneca Covid-19 vaccine that the Vietnamese government plans to purchase has certain edges over other candidates.
Oxford – AstraZeneca vaccine is reasonably priced at US$3-4 per dose, while Pfizer-BioNTech and Moderna vaccine is respectively charged at US$20 and US$30 per dose.
On top of that, the vaccine is easily delivered given the fact that it only requires storage temperatures from 2-8 degrees Celcius and can last for 6 months inside the normal medical refrigerator.
At the other end of the spectrum, the Pfizer-BioNTech vaccine must be stored and transported at minus 70 degrees Celcius and kept in the normal medical refrigerator at 2-8 degrees C for up to only 5 days.
 | |
|
| Vietnam’s Ministry of Health (MOH) on January 30 authorized the use of UK’s Oxford-Astra Zeneca vaccine in COVID-19 treatment. The country is expecting 50,000 doses in the First Quarter of 2021. According to Thanh Nien, the MOH’s Advisory Council for the Grant of Circulation Registration Numbers for Drugs granted Oxford-Astra Zeneca vaccine the circulation permit for emergency prevention, anti-epidemic in Vietnam. Vietnam is expected to receive the first 50,000 Oxford-Astra Zeneca doses in the first three months into 2021. The UK-based pharmaceutical company has earlier committed to providing Vietnam around 30 million doses in 2021. The MOH is also negotiating with other vaccine manufacturers with potential candidates including Pfizer and Moderna, hoping to purchase more COVID-19 vaccines for Vietnam. |
Vaccines development around the world
Russia is the world’s first nation to submit its vaccine Sputnik V for emergency approval since the pandemic broke out. The country has started vaccination program as soon as August on over 100,000 residents. On November 24, Sputnik V was pronounced with 91.4 percent effective.
In late November, US’s Moderna also announced their Covid-19 vaccine candidate to be 94.1 percent. Two vaccine manufacturers Johnson & Johnson and Novavax are planning to release their trial data in early 2021.
On December 3, the UK became the first nation in the world to ever roll out its Pfizer-BioNTech vaccine for mass vaccination. The US, Canada, Singapore, Baren, Mexico, France and EU had followed suit. Pfizer-BioNTech vaccine is over 90 percent effective though, its harsh storing and transport requirements (minus 70 degrees Celcius) make a big drawback for the candidate.
 | Intense Covid's vaccines dispute between UK and EU continues, revealing the truth about vaccine nationalism EU and UK's dispute over Covid-19's vaccine deliveries has become more intense, and whether contract means Covid jabs produced in UK must be sent to ... |
 | China and India's global influence to bolster due to Covid-19 vaccination campaigns With mass vaccination campaigns fighting Covid-19 underway globally, India and China has started to develop, produce and transport vaccines to the developing world, which will ... |
 | Oxford-Astra Zeneca to become first authorized COVID-19 vaccine in Vietnam Vietnam’s Ministry of Health (MOH) on January 30 authorized the use of UK’s Oxford-Astra Zeneca vaccine in COVID-19 treatment. The country is expecting 50,000 doses ... |
Recommended
 World
World
Vietnam’s 50-year Reunification Celebration Garners Argentine Press’s Attention
 World
World
"Will continue offering our full support to Indian govt": US FBI Director after Pahalgam attack
 World
World
"Great Leader": JD Vance Lauds PM Modi During His India Visit
 World
World
Trump’s Tariff Pause: A Strategic Move from “The Art of the Deal”?
 World
World
"Indian Navy's participation in AIKEYME exercise matter of great happiness": Admiral Dinesh Kumar Tripathi
 World
World
ASEAN and US Tariff Dilemma: Hybrid Approach to Global Trade Tensions
 World
World
Vietnam Affirms Its Active and Responsible Role at UNESCO
 World
World