Russian university successfully completes trials of world’s 1st Covid-19 vaccine
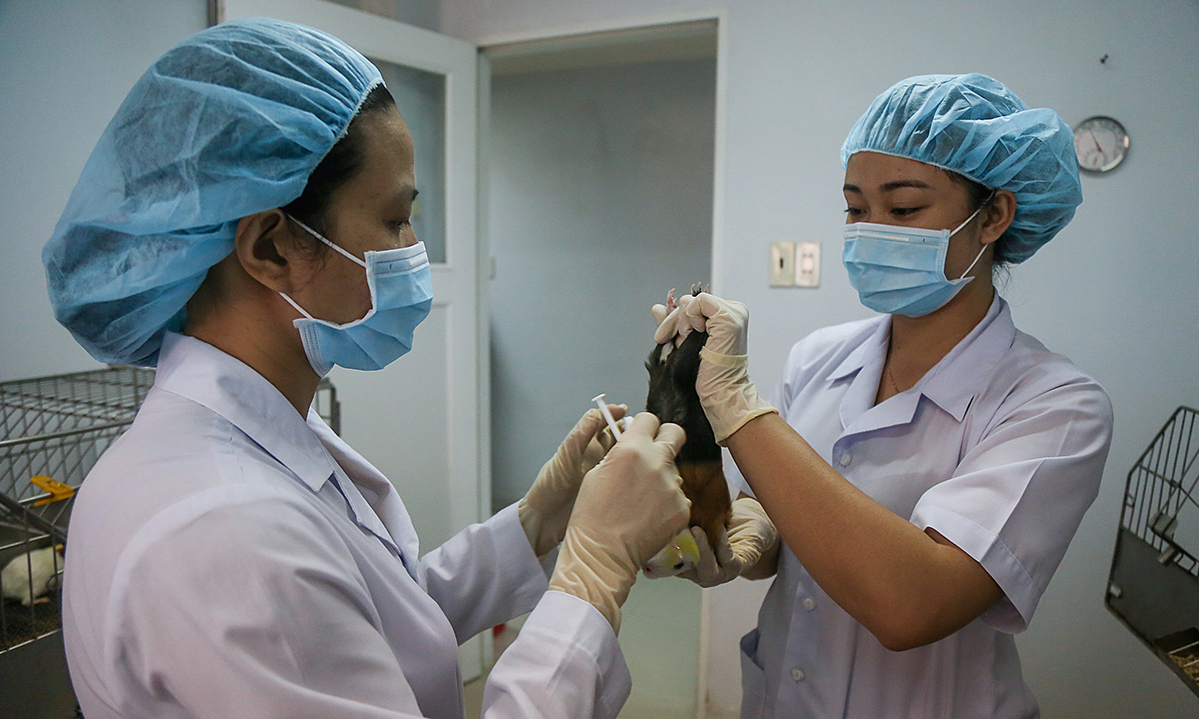
 |
| All clinical trials of COVID-19 vaccine produced by Gamalei Institute of Epidemiology and Microbiology started on June 18. Photo: Reuters |
"The clinical trials of the world’s first coronavirus vaccine on volunteers at Sechenov First Moscow State Medical University have been successfully completed", Vadim Tarasov, the director of the Institute for Translational Medicine and Biotechnology, told Sputnik, adding that the first group of volunteers would be discharged on Wednesday and the second on July 20.
The university began clinical trials of the vaccine produced by Russia’s Gamalei Institute of Epidemiology and Microbiology on June 18.
“Sechenov University has successfully completed tests on volunteers of the world’s first vaccine against coronavirus,” Tarasov said.
According to Alexander Lukashev, the director of the Institute of Medical Parasitology, Tropical and Vector-Borne Diseases at Sechenov University, the objective of this stage of the study was to show the vaccine’s safety for human health, which was successfully done.
“The safety of the vaccine is confirmed. It corresponds to the safety of those vaccines that are currently on the market,” Lukashev told Sputnik.
 |
| The clinical trials of the world’s first coronavirus vaccine on volunteers at Sechenov First Moscow State Medical University has been successfully completed. Photo: hindustantimes |
The further vaccine development plan is already being determined by the developer’s strategy, including the complexity of the epidemiological situation with the virus and the possibility of scaling up production, Lukashev added.
“Sechenov University in a pandemic situation acted not only as an educational institution but also as a scientific and technological research center that is able to participate in the creation of such important and complex products as drugs ... We worked with this vaccine, starting with preclinical studies and protocol development, and clinical trials are currently underway,” Tarasov noted.
"The vaccine is given twice with the same gene injected using different carriers, which allows to not just get protective immunity, but to acquire it for a longer period of time," Gintsburg said in an interview with Krasnaya Zvezda, the official newspaper of the Russian Defense Ministry. He added that such an approach "guarantees with great probability that a person getting this vaccine in the booster form will be protected from the coronavirus infection for at least two years, maybe even for a longer period of time", the director of the national research center, Alexander Gintsburg, said.
According to Gintsburg, about 50-60 million doses of the vaccine, or maybe even 70 million, will need to be produced in order to carry out mass coronavirus vaccination in Russia.
Russia reportedly completes world's first COVID-19 vaccine human trials. Video: Arirang
 |
| Russian military experts visit hospital facilities for elderly people to fight against the COVID-19 coronavirus infection, in Bergamo, Italy. Photo: SPUTNIK |
| Will the vaccine enter the commercial production stage? There was, however, no further information on when this vaccine would enter the commercial production stage. Russia had allowed clinical trials of two forms of a potential Covid-19 vaccine developed by the Gamaleya National Research Center for Epidemiology and Microbiology on June 18. The first vaccine, in the form of a solution for intramuscular administration, was carried out at the Burdenko Military Hospital. Another vaccine, in the form of a powder for the preparation of a solution for intramuscular administration, was carried out at Sechenov First Moscow State Medical University. The first stage of research on the vaccine at Sechenov University involved a group of 18 volunteers and the second group involved 20 volunteers. After vaccination, all volunteers were expected to remain in isolation in a hospital for 28 days. Vaccine tests performed on volunteers showed immunity to coronavirus Earlier, results of the COVID-19 vaccine tests performed on a group of volunteers in Russia showed that they were developing immunity to the coronavirus. "The data obtained by the Gamalei National Research Center for Epidemiology and Microbiology, proves that volunteers of the first and second groups are forming an immune response after injections of the vaccine against the coronavirus," according to an earlier statement from the Russian Defense Ministry.
|
| As the most updated announcement by The Moscow Times on July 12, Russia has reported 727,162 cases and 11,335 deaths to date. There are at least 21 vaccines currently under key trials, according to the World Health Organisation (WHO). According to the Moscow Times, Russia will begin negotiations with other countries to restart international flights from July 15. Flights will be permitted to a specific list of countries where cases of the coronavirus do not exceed 40 per 100,000 people, where the average daily increase in new cases is no higher than 1%, and where both countries agree to resume air connections. India TV News citing from a source of Johns Hopkins University in the US revealed that, the overall number of global COVID-19 cases was nearing 12.7 million, while the deaths have increased to more than 564,000. As of Sunday morning, the total number of cases stood at 12,681,472, while the fatalities rose to 564,420. The US accounted for the world's highest number of infections and fatalities at 3,245,158 and 134,764. Brazil came in the second place with 1,839,850 infections and 71,469 deaths.
|
 | International organizations seek cooperation with Vietnam in COVID-19 vaccine producing In the afternoon of June 30, Vietnam Deputy Prime Minister Vu Duc Dam, Head of the National Steering Committee on COVID-19 Prevention and Control, presided ... |
 | India: First COVID-19 vaccine gets approval for human trials Permission was granted after the firm submitted results from pre-clinical studies of the vaccine that demonstrated its safety and immune response, said Bharat Biotech. |
 | COVID 19 Vaccines available soon are ”Made-in-Vietnam” “Made-in-Vietnam” vaccines against COVID-19, which is developed by the State-owned Company for Vaccine and Biological Production No.1, and sponsored by the Vingroup Innovation Foundation- VinIF ... |
Recommended
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World