India: First COVID-19 vaccine gets approval for human trials
 |
India’s first COVID-19 vaccine ‘COVAXIN’ gets DCGI approval for clinical trials. Photo: Times Now News |
COVAXIN, India’s first vaccine candidate against the novel coronavirus, developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV), has received approval from the Drug Controller General of India (DCGI) to conduct Phase I and 2 human trials. According to the vaccine maker, human clinical trials of the experimental COVID-19 are scheduled to start across the country in July 2020, reported Times Now.
The SARS-CoV-2 strain was isolated in NIV, Pune, and transferred to Bharat Biotech. The indigenous, inactivated vaccine has been developed and manufactured at Bharat Biotech’s BSL-3 (Bio-Safety Level 3) High Containment facility located in Genome Valley, Hyderabad, India, the firm said in a release on Monday (June 29).
The Drug Controller General of India - CDSCO, Ministry of Health & Family Welfare, granted permission to initiate Phase I and II human trials after the company submitted results generated from preclinical studies, demonstrating safety and immune response.
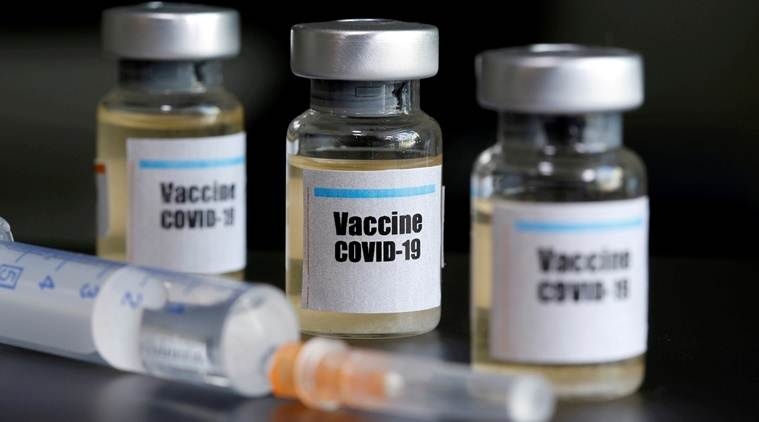 |
| Drug Controller General of India Dr V G Somani approved Bharat Biotech’s application to conduct phase I and II clinical trials for Covaxin. Photo: Indian Express |
“We are proud to announce COVAXIN, India’s first indigenous vaccine against COVID-19. The collaboration with ICMR and NIV was instrumental in the development of this vaccine. The proactive support and guidance from CDSCO have enabled approvals to this project. Our R&D and Manufacturing teams worked tirelessly to deploy our proprietary technologies towards this platform,” said Dr Krishna Ella, Chairman and Managing Director, Bharat Biotech, announcing the vaccine development milestone.
According to Times Now, the company accelerated its objective in completing the comprehensive preclinical studies. Results from these studies have been promising, showing extensive safety and effective immune responses.
 |
| More and more new vaccines for COVID-10 introduced these days. Photo: Reuters |
“Our ongoing research and expertise in forecasting epidemics has enabled us to successfully manufacture a vaccine for the H1N1 pandemic. Continuing our focus on creating the only BSL-3 containment facilities for manufacturing and testing in India, Bharat Biotech is committed to advancing vaccine development as a matter of national importance to demonstrate India’s strength in handling the future pandemics,” said Mrs Suchitra Ella, Joint Managing Director.
Bharat Biotech’s track record in developing Vero cell culture platform technologies has been proven in several vaccines for polio, rabies, rotavirus, Japanese Encephalitis, Chikungunya and Zika.
Apart from Covaxin, Bharat Biotech is already partnering with US-based vaccine maker FluGen and virologists at the University of Wisconsin-Madison to develop an intranasal vaccine - CoroFlu. It has also inked an exclusive deal with the Thomas Jefferson University of Philadelphia for the development of a new vaccine candidate for Covid-19, which has been invented at Jefferson using an existing deactivated rabies vaccine as a vehicle for coronavirus proteins, reported Times of India.
According to the Health Ministry, India’s total coronavirus cases reached 5,48,318 as of Monday evening. Worldwide, the novel coronavirus has now claimed at least 501,847 lives and infected as many as 10,161,240 people.
COVAXIN, India's First COVID-19 Vaccine Candidate, Set For Phase I, II Human Trials. Video: NDTV
 | Vietnam to continue testing COVID-19 vaccine on mice Company for Vaccine and Biological Production 1 (VABIOTECH) under the Ministry of Health will conduct a second test of Covid-19 vaccine on mice next month, ... |
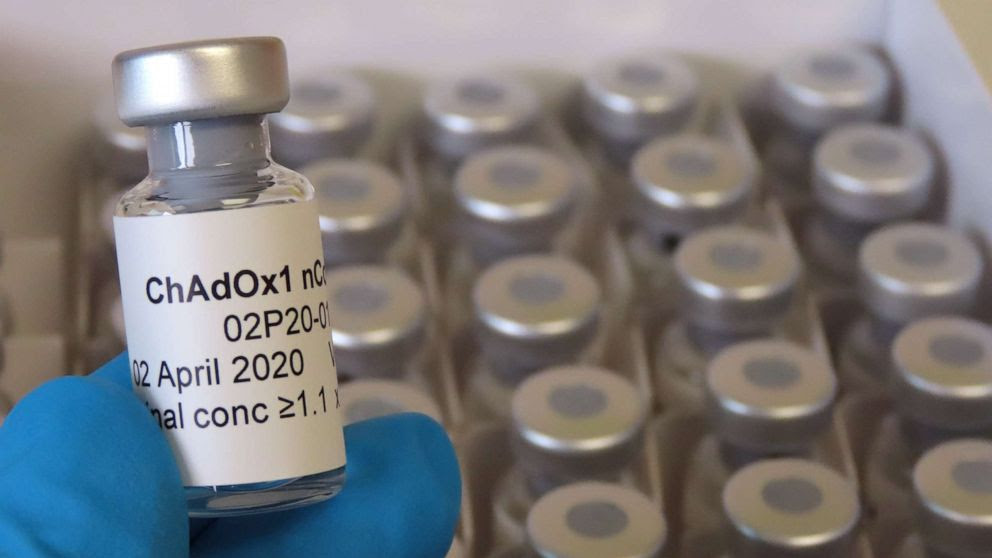 | A landmark partnership announced for development of COVID-19 vaccine in the UK The University of Oxford has announced an agreement with the UK-based global biopharmaceutical company AstraZeneca, headquarter in the UK and representative office in Vietnam, for ... |
 | Oxford-developed vaccine appears to shield monkey from coronavirus infection Oxford group may lead the race in coronavirus vaccine development as its new experimental vaccine seems to protect monkeys from the novel acute respiratory virus despite ... |
Recommended
 World
World
India reports 9 Pakistani Aircraft Destroyed In Operation Sindoor Strikes
 World
World
Thailand Positions Itself As a Global Wellness Destination
 World
World
Indonesia Accelerates Procedures to Join OECD
 World
World
South Korea elects Lee Jae-myung president
Popular article
 World
World
22nd Shangri-La Dialogue: Japan, Philippines boost defence cooperation
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World








![[Photos] India and Vietnam Unite in Friendship Festival 2024](https://vietnamtimes.org.vn/stores/news_dataimages/2024/122024/19/23/b72443a0d3e09c7d133e23a7d221ca07.jpg?rt=20241219234711)

