UK is world’s first country to approve Pfizer’s COVID-19 vaccine
| What is mRNA-1273 - Moderna COVID-19 vaccine? | |
| Gold price hits 4-month low as vaccine progress slams precious metals | |
| COVID-19 vaccines could get green light from EU regulator late 2020, early 2021 |
 |
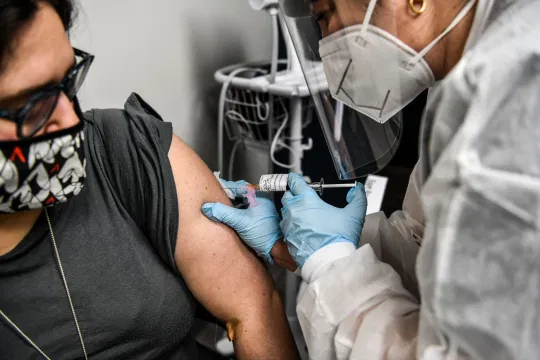
| With the authorization, citizens outside of the worldwide clinical trials will have the opportunity to be immunized against COVID-19(Photo: Financial Times) |
This marks the first time that citizens outside of the worldwide clinical trials will have the opportunity to be immunized against Covid-19, according to BioNTech CEO Ugur Sahin.
The vaccine will be made available across the UK starting next week, a spokesperson from the Department of Health and Social Care said in a statement. More details will be released soon, including advice for priority groups like the elderly and health care staff to receive the vaccine, CNN reported.
"To aid the success of the vaccination programme it is vital everyone continues to play their part and abide by the necessary restrictions in their area so we can further suppress the virus and allow the NHS to do its work without being overwhelmed," the spokesperson said.
"For so long we've been saying that if a vaccine is developed, then things will get better in 2021, and now we can say when this vaccine is rolled out things will get better," Hancock told the BBC.
The U.K. has ordered 40 million vaccine doses from Pfizer — enough for up to a third of the population.
The vaccine was found to be 95 percent effective at preventing symptomatic Covid-19, the drugmaker said after clinical trials.
"The vaccine will be made available across the UK from next week. The NHS has decades of experience in delivering large scale vaccination programmes and will begin putting their extensive preparations into action to provide care and support to all those eligible for vaccination," the statement added.
Russia and China have already approved vaccines without waiting for the results of large-scale efficacy tests, a decision that scientists in some cases have said poses serious risks.
While the go-ahead bodes well for Britain, which broke from the European Union’s regulatory orbit to approve the shot early, it will have no effect on the distribution of the hundreds of millions of doses that other wealthy countries have procured in prepaid contracts.
It also offers little relief to poorer countries that could not afford to buy supplies in advance and may struggle to pay for both the vaccines and the exceptional demands of distributing them, as reported by NY Times.
In a news release, Pfizer CEO Albert Bourla hailed the emergency authorization as "a historic moment in the fight against Covid-19."
"This authorization is a goal we have been working toward since we first declared that science will win, and we applaud the (Medicines & Healthcare Products Regulatory Agency) for their ability to conduct a careful assessment and take timely action to help protect the people of the UK," he said.
Sahin added that the regulatory data was the result of "a scientifically rigorous and highly ethical research and development program."
 |
| Vials of the coronavirus vaccine at the Pfizer manufacturing facility in Kalamazoo, Michigan. (Photo: Reuters) |
The pharmaceutical giant submitted an application to the Food and Drug Administration on Nov. 20 for an emergency use authorization in the U.S.
A vaccinecommittee will now decide which groups will first get the vaccine, such as care home residents, health and care staff, the elderly and people who are clinically vulnerable.
| The Pfizer shots must be stored at minus 94 degrees Fahrenheit — far colder than standard cooling systems. To help accommodate the extra refrigeration requirement, Pfizer has developed a supercool storage unit packed with dry ice. Those requirements, along with high costs, could substantially limit the number of countries and people that have access to those vaccines. For that reason, much of the world had been waiting expectantly for the results from AstraZeneca and Oxford, which will cost only a few dollars per dose and is easy to store for long periods. Britain, too, has staked a considerable part of its vaccine strategy on the AstraZeneca vaccine, buying 100 million doses, NY Times said. |
| Nearly 1.5 million people around the world have died from the virus, with more than 271,000 deaths in the U.S. and nearly 60,000 deaths in the U.K. Other countries, including the United States, are considering approval for various coronavirus vaccines before the end of the year. |
 | WHO warns vaccines won’t help immediately fend off COVID-19 infections The World Health Organization on Wednesday warns that a coronavirus vaccine won’t help countries beat back the current wave of COVID-19 vaccine sweeping across Europe, ... |
 | Vietnam to test COVID-19 vaccine on the elderly The elderly people are target subjects of the prevention and control of the COVID-19 pandemic. All COVID-19 vaccine developers and manufacturers need to prove the ... |
 | World breaking news today (November 19): Pfizer's Covid-19 vaccine shows 95 percent effective World breaking news today (November 19): Pfizer's Covid-19 vaccine shows 95 percent effective. Meanwhile, Central America flooding wreaks havoc, U.S. COVID-19 deaths surpass 250,000 and ... |
Recommended
 World
World
Thailand Positions Itself As a Global Wellness Destination
 World
World
Indonesia Accelerates Procedures to Join OECD
 World
World
South Korea elects Lee Jae-myung president
 World
World
22nd Shangri-La Dialogue: Japan, Philippines boost defence cooperation
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World