COVID-19 treatment: South Korea, India authorise emergency use of remdesivir
 |
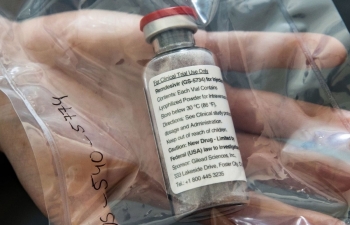
| (Photo: The Financial Express) |
Remdesivir, which is administered intravenously in hospital, is the first drug to show improvement in COVID-19 patients in formal clinical trials.
"Remdesivir can help reduce the amount of coronavirus in the body," South Korea's Ministry for Food and Drug Safety said in a statement. "This can help the patient's condition improve faster."
On Monday, Gilead reported the drug provided a modest benefit in patients with moderate COVID-19 given a five-day course of the treatment, while those who received the medicine for 10 days in the study did not fare as well.
Under guidelines announced by South Korea's Ministry for Food and Drug Safety, doctors can administer one dose of remdesivir a day, with 5 doses overall for patients with moderate symptoms, and 10 doses for patients with severe symptoms and who need oxygen support.
The ministry said it will cooperate with Gilead, the Korea Centers for Disease Control (KCDC) and Prevention and other ministries to swiftly import the drug, according to Reuters.
All patients must undergo a liver function test before taking the drug as possible side effects include elevated levels of liver enzymes, the ministry added,
On June 2, India ’s government has also approved Gilead Sciences Inc’s antiviral drug remdesivir for emergency use for five doses in treating COVID-19 patients.
The DCGI decided against extending its use to 10 days, based on existing evidence presented to it at the time of approval, the Indian Express newspaper had reported.
 |
| (Photo: Daily hunt) |
The US Food and Drug Administration granted emergency use authorization last month, citing results from a US government study that showed the drug reduced hospitalization stays by 31 percent, or about four days, compared to a placebo.
The US is the first country in the world to authorize the experimental anti-viral drug remdesivir to treat patients with COVID-19. The authorization was announced by President Donald Trump on May 2. The move is called by Gilead chief executive Daniel O'Day “ a significant step towards easing the coronavirus crisis”, abc.net.au reported.
Three days after the U.S. drugmaker filed for fast-track approval for the treatment, Japan became the second country to authorize remdesivir to treat coronavirus patients.
“There has so far been no coronavirus medicine available here so it is a significant step for us to approve this drug,” Reuters quoted a Japanese health ministry official said at a press briefing. Remdesivir will be given to patients with severe COVID-19 symptoms, he added.
Prime Minister Shinzo Abe said the government was getting ready to give a speedy green light to the experimental drug developed by U.S. firm Gilead Sciences, Japan Today reported.
| South Korea has been battling small but steady new outbreaks of COVID-19, with 49 new cases reported on Tuesday, bringing the country's total to 11,590 cases with 273 deaths. Last Thursday, South Korea re-imposed a series of COVID-19 social distancing measures it had eased early this month, as a series of clusters threatened to challenge its success in containing the epidemic. Museums, parks and art galleries were closed from last Friday for two weeks, while companies were urged to re-introduce flexible working, among other measures, Reuteres reported. Meanwhile, India’s coronavirus infections crossed 200,000, the latest official figures showed, with the death toll at 5,815. |
 | 78 percent of hotels and resorts in Vietnam resume operation post-Covid-19 78 percent of four- and five-star hotels and resorts across Vietnam have recommenced operations following months of closure due to the novel coronavirus. |
 | Taiwan considers shortened Covid-19 quarantine for Vietnamese arrivals Thanks to its "low-risk" Covid-19 status, travelers from Vietnam will be quarantined only five days instead of 14 on entering Taiwan. |
 | Serving COVID-19 US doctors free meals, Vietnamese American promotes Vietnamese cuisine Phan Tieu Van, a Vietnamese American, has called for thousands of meals for medics at hospitals across North California during the lock-down period. Her good ... |
In topics
Recommended
 World
World
Pakistan NCRC report explores emerging child rights issues
 World
World
"India has right to defend herself against terror," says German Foreign Minister, endorses Op Sindoor
 World
World
‘We stand with India’: Japan, UAE back New Delhi over its global outreach against terror
 World
World
'Action Was Entirely Justifiable': Former US NSA John Bolton Backs India's Right After Pahalgam Attack
Popular article
 World
World
US, China Conclude Trade Talks with Positive Outcome
 World
World
Nifty, Sensex jumped more than 2% in opening as India-Pakistan tensions ease
 World
World
Easing of US-China Tariffs: Markets React Positively, Experts Remain Cautious
 World
World