First Domestic Covid-19 Vaccine May Be Approved For Emergency Use
 |
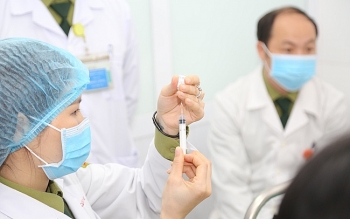
| Photo: VOV |
On Thursday afternoon, the Ministry of Health and the Ministry of Science and Technology convened a meeting to review and evaluate the outcomes of clinical trials of Nanocovax produced by Nanogen Pharmaceutical Biotechnology JSC (Nanogen) based on recombinant DNA/protein technology.
Based on the evaluation outcomes, the vaccine may be approved for emergency use in the country, VGP cited.
It went through the first phase trial from December 18, 2020, and the second phase from February 26, 2021. The third phase started on June 11, 2021. Results from the first two trial phases showed that all volunteers developed antibodies against SARS-CoV-2.
At the meeting, a representative from Nanogen said more than 13,620 volunteers had been vaccinated with the Nano Covax vaccine so far. The third and the final phase saw the participation of 13,000 volunteers with 1,004 people getting two jabs, according to Vietnam News.
 |
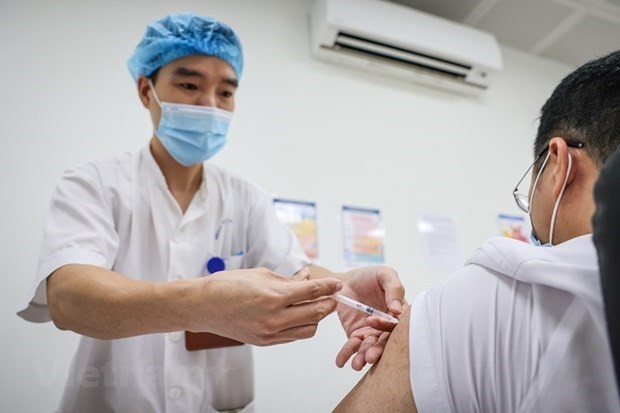
| Photo: VNA |
The company said it would continue to complete documents relating to the products and outcomes of the trials to submit to the Ministry of Health next week.
Chairman of the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients Lê Văn Truyền said the council supported the research and development of homegrown vaccines but registration dossiers for approving vaccines in emergency situations must meet scientific and legal standards.
Deputy Minister of Science and Technology Bui The Duy said under the current complex development of the pandemic, the ministry hoped health experts would evaluate outcomes of the clinical trials scientifically and create conditions to speed up research and production of the vaccine.
Deputy Minister of Health Tran Van Thuan said the ministry supported and created conditions for Nanogen as well as other domestic companies and units to research and develop vaccines with the hope that Việt Nam would soon be able to produce “Made in Vietnam” vaccines to help ensure vaccine supply for coronavirus control.
Regarding licensing conditions, Thuan asked Nanogen to work with agencies to complete dossiers about the outcomes of the first and second phases of human trials and soon update the results of the third phase.
 |
| Photo: Saigon Times |
Based on the outcomes of trials and the opinions of domestic and foreign experts as well as the pandemic situation and vaccine demand, the Ministry of Health will study and consider the licensing of the Nano Covax vaccine for emergency use.
Nanocovax is currently the most promising candidate among four domestic vaccines being developed by Viet Nam. The others are being developed by the Institute of Vaccines and Medical Biologicals, the Vaccine, and Biological Production Company No. 1, and the Center for Research and Production of Vaccines and Biologicals. The price for each dose was projected to be VND 120,000 (US$ 5.2). The doses are given intramuscularly with the spacing between each dose about a month, according to Nanogen.
Viet Nam has approved six Covid-19 vaccines for emergency use, namely Janssen, Moderna, Sputnik V, Pfizer, Sinopharm, and AstraZeneca.
The Government of Viet Nam is making every effort to secure at least 150 million vaccine doses to cover 70% of the population.
So far, Viet Nam has received more than 8 million doses of Covid-19 vaccines from other countries and partners, including 4.5 million doses through the COVAX Facility, 2 million doses donated by the United States, 3 million doses from Japan, 500.000 doses by China, and 1.000 doses donated by the Russian Federation.
 | Russian Ambassador: Russia Ready To Transfer Vaccine Production Technology To Vietnam Russia is ready to provide Covid-19 vaccines and transfer vaccine production technology to Vietnam, said Gennady Bezdetko, newly-appointed Russian ambassador to Vietnam, on Thursday. |
 | A Look into Sputnik V Processing Line in Vietnam The first batch of the Sputnik V vaccine has been produced in Vietnam under a seven-step process before being sent to Russia for inspection. |
 | Vietnam Government supports T&T Group in purchase of 40 million Sputnik V vaccine doses HANOI, VIETNAM - Media OutReach - 22 July 2021 - On July 12, the Government of Vietnam issued Resolution No. 73/NQ-CP allowing T&T Group to ... |
In topics
Recommended
 Viet's Home
Viet's Home
Hue City to Raise Awareness on Mine Accident Prevention
 Focus
Focus
Vietnam Leaves Imprints on the World Peacekeeping Map
 Viet's Home
Viet's Home
“Global Vietnamese Singing 2025” - Connecting Hearts Longing for Homeland
 Viet's Home
Viet's Home
Vietnam’s People's Public Security Force Actively Contributes to UN Peacekeeping Operations
Popular article
 Viet's Home
Viet's Home
HAUFO Enhances Competence of People-to-People Diplomacy Personnel
 Viet's Home
Viet's Home
Hands that Reserve Da Long Brocade Craft
 Viet's Home
Viet's Home
Da Rsal – How Digital Transformation Reshape a Poor Commune
 Viet's Home
Viet's Home