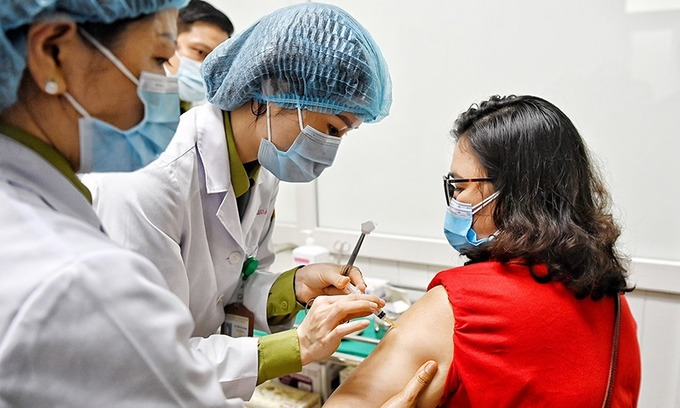
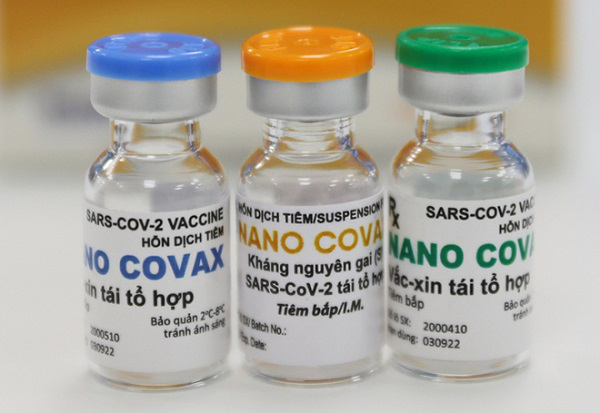
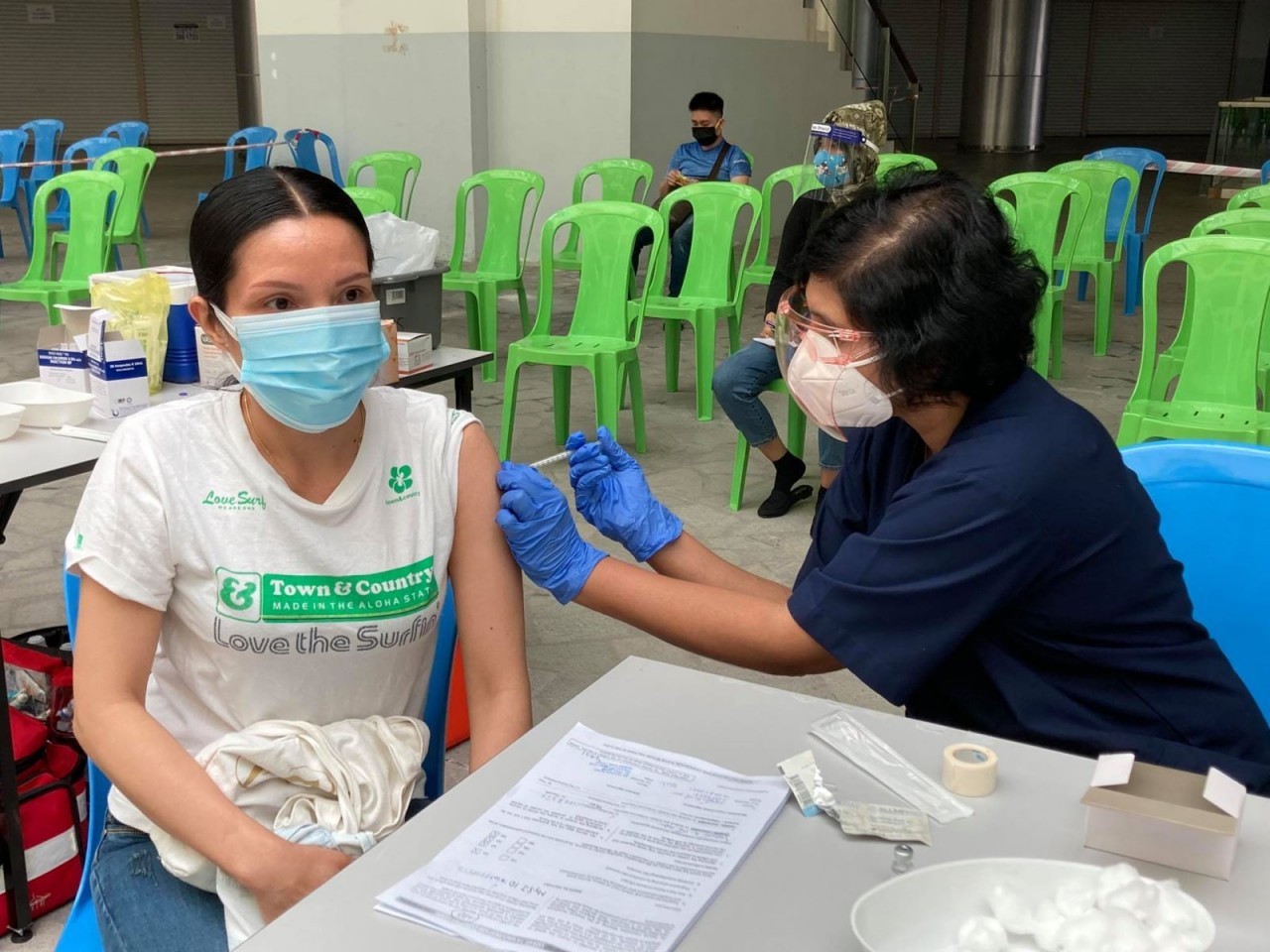
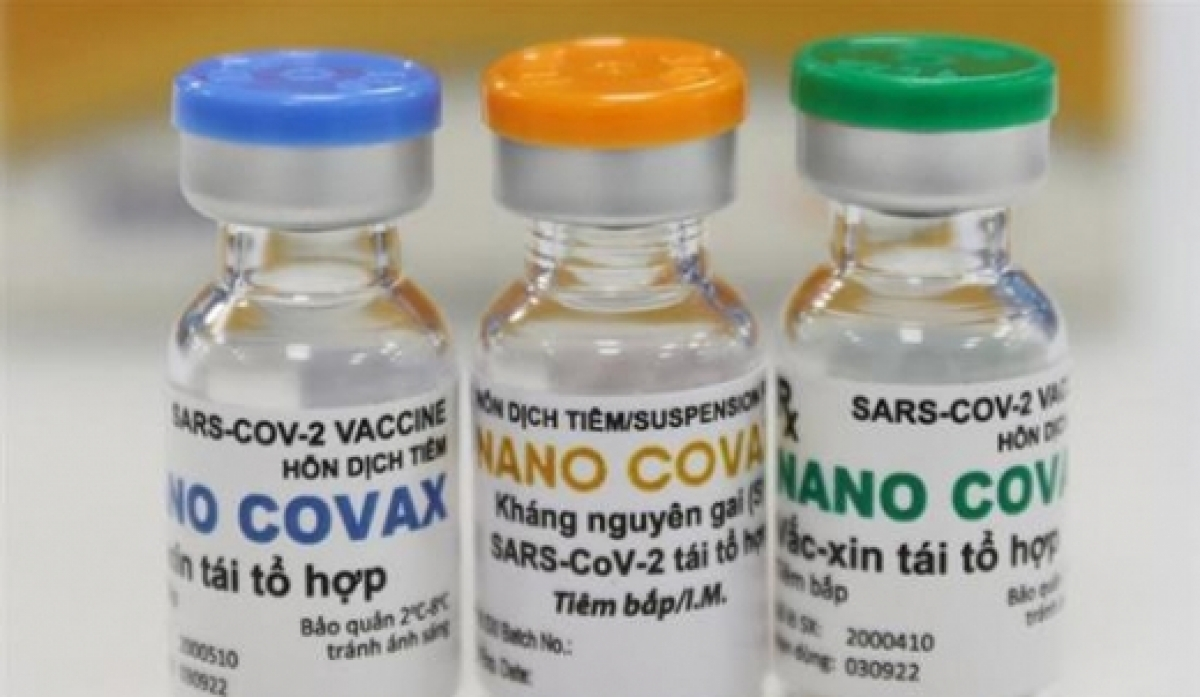
Positive developments with vaccines made in Vietnam
Nano Covax is Vietnam’s first Covid-19 vaccine tested on humans. The vaccine was developed by the Nanogen Pharmaceutical Biotechnology JSC, based on recombinant protein technology. The clinical trial of Nano Covax began officially in December 2020.
The third phase of the vaccine’s clinical trial is expected to be carried out in early June 2021 to test Nano Covax’s effectiveness. Volunteers for this phase will receive shots of 25mcg dose.
Three months after the second phase of the Nano Covax clinical trial, the volunteer’s level of SAR-CoV-2 antibodies has increased.
Scientists believed this positive sign is an important basis to speed up the third phase of Nano Covax testing.
Nano Covax is safe
The second phase of the Nano Covax clinal trial began on February 26.
Four groups of volunteers participated in the second phase: Three groups received shots of different doses (25mcg, 50mcg, 75mcg) and one group received placebos.
One of the 554 volunteers to be selected for the second phase, D.N.D, 45, from Hoang Mai district in Hanoi, said she and her family decided to partake in the trial after they learned about the program in early 2021.
She felt lucky to be eligible for becoming a volunteer for the second trial.
After receiving the vaccine, D. felt tired and her arm was slightly hurt around the injection area, but she did not have any fever. After three days, these symptoms disappeared.
Following the second shot, she underwent health evaluation once per week. D. has completely recovered now.
Another volunteer for the second phase of the Nano Covax trial, N.V.N, 34, from Tay Ho district, had researched thoroughly about the trial, including possible side effects, before taking part in the trial.
"I believe in Vietnam’s medical development, so I did not hesitate to register to be a volunteer. I want to help push back the pandemic," said N.
|
According to Doctor Ho Anh Son, Vice Director of the Institute of Biomedicine and Pharmacy, the second phase of the Nano Covax vaccine clinical trial is almost over. Volunteers will continue to be monitored until six months after the first shot.
To monitor the vaccine’s safety and effectiveness, Son said the same research procedures were adopted across all research sites: After receiving the shot, volunteers were monitored on-site for one hour. Afterward, they went home, took note of their health conditions every day, and brought this note to their next health evaluation session.
In the first few days, the medical staff contacted each volunteer to check and monitor their health conditions.
After the second injection, volunteers went through health check-ups once a week to evaluate the level of antibodies in their bodies. They would continue to be monitored for six months to assess their health conditions and immunity generation.
Assessing Nano Covax’s effectiveness and safety, Son said all volunteers who received the vaccine have generated an immune response. In the world, the best Covid-19 vaccines are 90% effective. Some only achieve an effective level of 60 – 70%.
"The number of SAR-CoV-2 antibodies in all volunteers has increased. The volunteer’s health was stable. Nobody had to take medication or seek immediate emergency medical care after being injected. This is encouraging news. At this point, Nano Covax is safe for the volunteers.” said Son.
13,000 people will join the third phase of the Nano Covax clinical trial
Son said the third phase of the Nano Covax trial is expected to be carried out in early June, with the participation of 13,000 people.
"The third phase will be carried out quickly while still adhering strictly to the standard procedures, meeting the needs to control Covid in Vietnam,” said Son.
Vietnam’s Military Medical University has been mobilizing a significant number of doctors, technicians, and medical students to carry out the third trial simultaneously in 10 provinces and cities.
The criteria for selecting volunteers have been loosened, following the assessment of the results from the first and second phases of the Nano Covax clinical trial.
Volunteers for the third phase need to be from 18 to 75 years old. They will undergo blood formulae and SARS-CoV-2 antibodies checking.
 |
| Photo: Testing Nano Covax / Ncov.moh.gov.vn |
"Those who have been exposed to SARS-CoV-2 or have antibodies will not be eligible,” said Son.
The third phase is designed to evaluate the vaccine’s ability to protect the community. It will be carried out in many medical centers and extended to other provinces. The 25mcg dose is selected for the third phase.
Volunteers will continue to be divided into groups to receive the vaccine and placebo. Health evaluation will be carried out after the first shot, after the second shot (injected 28 days after the first shot), and on the 35th and 42nd days after injection.
"In theory, these are ideal benchmarks to evaluate vaccine safety and immunity criteria. The results of the trial will be reported to the Research Ethics Committee. In case of emergency, the Ministry of Health may approve this vaccine for emergency use,” said Doctor Do Quyet – Director of the Military Medical Hospital.
 | Vietnamese-American share positive beliefs about COVID-19 vaccine Many Americans in general and Vietnamese-born Americans, in particular, are willing to vaccinate, putting their faith in the effectiveness of COVID-19 vaccines. |
 | First alternating dose vaccine trail launches in UK, promising new possibilities A new study being led by University of Oxford and run by the National Immunisation Evaluation Consortium suggests exploring whether using different Covid-19 vaccines for ... |
 | Updates on World COVID vaccines: China approves Sinopharm, UK authorizes AstraZeneca Chinese regulators have approved the country's first homegrown coronavirus vaccine, developed by state-owned pharmaceutical giant Sinopharm, while Health officials in the U.K. authorized the AstraZeneca-Oxford ... |
Recommended
 National
National
Vietnam News Today (May 31): Vietnam Strongly Supports Laos’s National Development
 National
National
Vietnam News Today (May 30): Vietnam, Venezuela Reinforce Ties Through People-to-people Diplomacy
 National
National
Vietnam News Today (May 29): Vietnam and Hungary to Expand Cooperation into New Areas
 National
National
Vietnam News Today (May 28): Vietnam and China Discuss Strategic Cooperation Orientations
Popular article
 National
National
Vietnam News Today (May 27): Vietnam Treasures Multifaceted Collaboration with France
 National
National
Vietnam Commits to Building an Inclusive, Sustainable and Cohesive ASEAN
 National
National
Vietnam Proposes Vision for Responsible Digital Journalism Cooperation
 National
National